Nitrofurantoin
Nitrofurantoin Capsules, USP (Monohydrate/Macrocrystals), 100 mg (Twice-a-day Dosage)
b644426c-c8ba-4fe0-80c7-d9b2dde37395
HUMAN PRESCRIPTION DRUG LABEL
Aug 13, 2025
A-S Medication Solutions
DUNS: 830016429
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Nitrofurantoin (monohydrate/macrocrystals)
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (23)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NITROFURANTOIN- MONOHYDRATE/MACROCRYSTALS

HOW SUPPLIED SECTION
HOW SUPPLIED
Product: 50090-6769
DESCRIPTION SECTION
DESCRIPTION
Nitrofurantoin is an antibacterial agent specific for urinary tract infections. Nitrofurantoin capsules, USP (monohydrate/macrocrystals) are hard gelatin capsule shells containing the equivalent of 100 mg of nitrofurantoin in the form of 25 mg of nitrofurantoin macrocrystals USP and 75 mg of nitrofurantoin monohydrate USP.
The chemical name of nitrofurantoin macrocrystals USP is 1-[[[5-nitro-2-furanyl]methylene]amino]-2,4-imidazolidinedione. The chemical structure is the following:
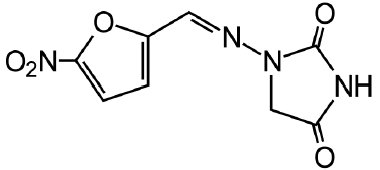
Molecular Weight: 238.16
The chemical name of nitrofurantoin monohydrate USP is 1-[[[5-nitro-2-furanyl]methylene]amino]-2,4-imidazolidinedione monohydrate. The chemical structure is the following:
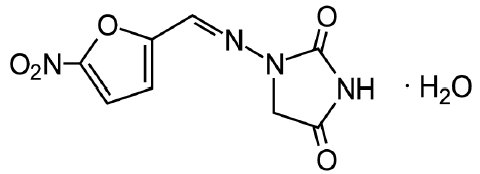
Molecular Weight: 256.17
Inactive Ingredients
Each capsule contains carbomer 934P, colloidal silicon dioxide, corn starch, compressible sugar, D&C yellow No. 10, edible white ink, FD&C blue No. 1, FD&C red No. 40, gelatin, lactose monohydrate, magnesium stearate, povidone, talc, and titanium dioxide.
Meets USP Dissolution Test 5
