Mint Alcohol Free
WinCo Foods 299.006/299AM MULTI-ACTION ALCOHOL-FREE ANTISEPTIC MOUTH RINSE WITH CPC
30b50da5-14d0-d0c7-e063-6394a90a091b
HUMAN OTC DRUG LABEL
May 22, 2025
WinCo Foods LLC
DUNS: 056098817
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Cetylpyridinium Chloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
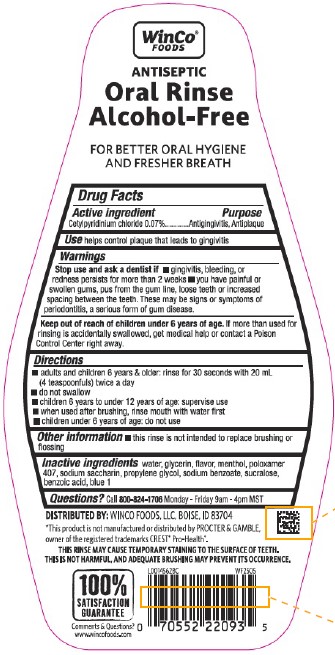
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
SEALED WITH PRINTED NECKBAND FOR YOUR PROTECTION
Compare to the active ingredient in CREST ®Pro-Health
WinCo ®
Foods
ANTISEPTIC
Oral Rinse
Alcohol-Free
ANTIGINGIVITIS/ANTIPLAQUE
MOUTH RINSE
Kills Germs Commonly Associated with Gingivitis
Helps Prevent Plaque
Freshens Breath
No Burn of Alcohol
Fresh Mint
1 L (1.05 QT) 33.8 FL OZ

INDICATIONS & USAGE SECTION
Use
helps control plaque that leads to gingivitis
ADVERSE REACTIONS SECTION
ADVERSE REACTIONS SECTION
DISTRIBUTED BY: WINCO FOODS, LLC, BOISE, ID 83704
100% SATISFACTION GUARANTEE
Comments & Questions?
www.wincofoods.com
SPL UNCLASSIFIED SECTION
Disclaimers
*This product is not manufactured or distributed by PROCTER & GAMBLE, owner of the registered trademarks CREST ®Pro-Health ®.
THIS RINSE MAY CAUSE TEMPORARY STAINING TO THE SURFACE OF TEETH.
THIS IS NOT HARMFUL, AND ADEQUATE BRUSHING MAY PREVENT ITS OCCURRENCE.
OTC - ACTIVE INGREDIENT SECTION
ACTIVE INGREDIENT
Cetylpyridinium chloride 0.07%
OTC - PURPOSE SECTION
PURPOSE
Antigingivitis, Antiplaque
WARNINGS SECTION
Warnings
for this product
OTC - STOP USE SECTION
Stop use and ask a dentist if
- gingivitis, bleeding, or redness persists for more than 2 weeks
- you have painful or swollen gums, pus from the gum line, loose teeth or increased spacing between the teeth. These may be signs or symptoms of periodontitis, a serious form of gum disease.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children under 6 years of age.
If more than used for rinsing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
Directions
- adults and children 6 years & older: rinse for 30 seconds with 20 mL (4 teaspoonfuls) twice a day
- do not swallow
- children 6 years to under 12 years of age: supervise use
- when used after brushing, rinse mouth with water first
- children under 6 years of age: do not use
OTHER SAFETY INFORMATION
Other information
- this rinse is not intended to replace brushing or flossing
INACTIVE INGREDIENT SECTION
Inactive ingredients
water, glycerin, flavor, menthol, poloxamer 407, sodium saccharin, propylene glycol, sodium benzoate, sucralose, benzoic acid, blue 1
OTC - QUESTIONS SECTION
Questions?
Call 800-824-1706 Monday - Friday 9am - 4pm MST
