Fensolvi
These highlights do not include all the information needed to use FENSOLVI safely and effectively. See full prescribing information for FENSOLVI.
2c311700-edf4-4f2a-a468-9875489a6cc7
HUMAN PRESCRIPTION DRUG LABEL
Apr 29, 2023
TOLMAR Inc.
DUNS: 791156578
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Leuprolide Acetate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (3)
Drug Labeling Information
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Initial Rise of Gonadotropins and Sex Steroid Levels
During the early phase of therapy, gonadotropins and sex steroids rise above baseline because of the initial stimulatory effect of the drug [see Clinical Pharmacology (12.2)]. Therefore, an increase in clinical signs and symptoms of puberty including vaginal bleeding may be observed during the first weeks of therapy or after subsequent doses [see Adverse Reactions (6)]. Instruct patients and caregivers to notify the physician if these symptoms continue beyond the second month after FENSOLVI administration.
5.2 Psychiatric Events
Psychiatric events have been reported in patients taking GnRH agonists, including leuprolide acetate. Postmarketing reports with this class of drugs include symptoms of emotional lability, such as crying, irritability, impatience, anger, and aggression. Monitor for development or worsening of psychiatric symptoms during treatment with FENSOLVI [see Adverse Reactions (6.1, 6.2)].
5.3 Convulsions
Postmarketing reports of convulsions have been observed in patients receiving GnRH agonists, including leuprolide acetate. These included patients with a history of seizures, epilepsy, cerebrovascular disorders, central nervous system anomalies or tumors, and patients on concomitant medications that have been associated with convulsions such as bupropion and SSRIs. Convulsions have also been reported in patients in the absence of any of the conditions mentioned above [see Adverse Reactions (6.2)].
5.4 Pseudotumor Cerebri (Idiopathic Intracranial Hypertension)
Pseudotumor cerebri (idiopathic intracranial hypertension) have been reported in pediatric patients receiving GnRH agonists, including leuprolide acetate. Monitor patients for signs and symptoms of pseudotumor cerebri, including headache, papilledema, blurred vision, diplopia, loss of vision, pain behind the eye or pain with eye movement, tinnitus, dizziness, and nausea [see Adverse Reactions (6.2)].
- Initial Rise of Gonadotropins and Sex Steroid Levels: During the early phase of therapy, gonadotropins and sex steroids rise above baseline because of the initial stimulatory effect of the drug. Therefore, an increase in clinical signs and symptoms of puberty including vaginal bleeding may be observed during the first weeks of therapy or after subsequent doses. Instruct patients and caregivers to notify the physician if these symptoms continue beyond the second month after FENSOLVI administration. (5.1)
- Psychiatric events: Have been reported in patients taking GnRH agonists. Events include emotional lability, such as crying, irritability, impatience, anger, and aggression. Monitor for development or worsening of psychiatric symptoms. (5.2)
- Convulsions: Have been observed in patients with or without a history of seizures, epilepsy, cerebrovascular disorders, central nervous system anomalies or tumors, and in patients on concomitant medications that have been associated with convulsions. (5.3)
- Pseudotumor Cerebri (Idiopathic Intracranial Hypertension): Have been reported in pediatric patients receiving GnRH agonists, including leuprolide acetate. Monitor patients for headache, papilledema, and blurred vision. (5.4)
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Information
FENSOLVI must be administered by a healthcare provider.
The dose of FENSOLVI is 45 mg administered by subcutaneous injection once every six months.
Discontinue FENSOLVI treatment at the appropriate age of onset of puberty.
2.2 Monitoring
Monitor response to FENSOLVI with a GnRH agonist stimulation test, basal serum luteinizing hormone (LH) levels or serum concentration of sex steroid levels at 1 to 2 months following initiation of therapy and as needed to confirm adequate suppression of pituitary gonadotropins, sex steroids, and progression of secondary sexual characteristics. Measure height (for calculation of growth velocity) every 3 to 6 months and monitor bone age periodically.
Noncompliance with drug regimen or inadequate dosing may lead to gonadotropins and/or sex steroids increasing above prepubertal levels resulting in inadequate control of the pubertal process. If the dose of FENSOLVI is not adequate, switching to an alternative GnRH agonist for the treatment of CPP with the ability for dose adjustment may be necessary.
2.3 Reconstitution Instructions
Use aseptic technique including gloves for reconstitution and administration. Allow the product to reach room temperature before reconstitution to allow for easier administration. Once reconstituted, the concentration is 45 mg/0.375 mL. Administer the product within 30 minutes or discard.
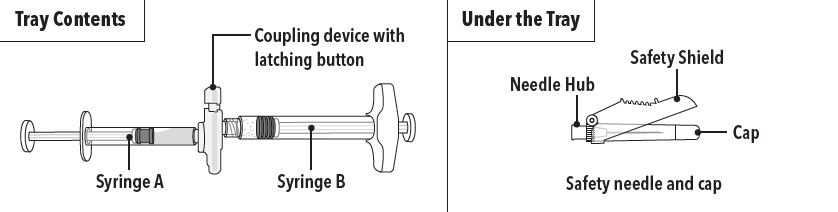
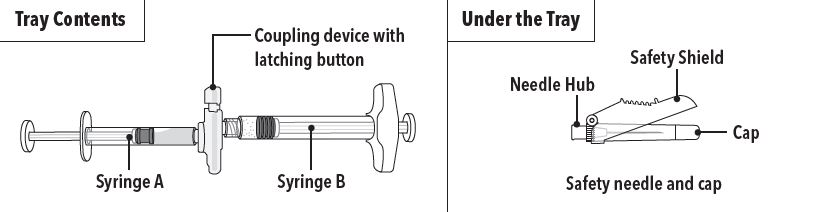
FENSOLVI is packaged in a carton containing:
- Tray containing pre-connected syringe system and desiccant pack
- Prescribing information
- Sterile safety needle and cap (located under the tray in carton)
Follow the detailed instructions below to ensure correct preparation of FENSOLVI prior to administration:
|
Step 1 On a clean field open the tray by tearing off the foil from the corner and remove the contents. Discard the desiccant pack. Remove the pre-connected syringe system from the tray. Open the sterile safety needle package by peeling back the paper tab.**Note:**Syringe A and Syringe B should not be lined-up yet. The product should only be administered with the co-packaged, sterile safety needle. | |
|
| |
|
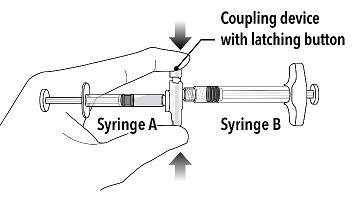
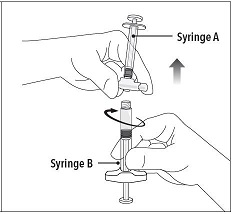
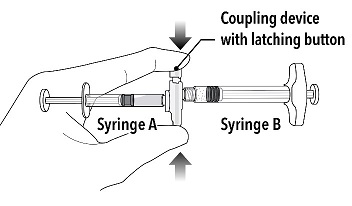
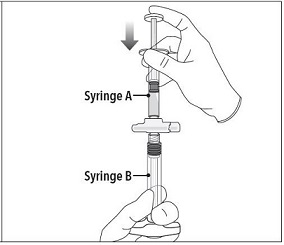
Step 2 Grasp the latching button on the coupling device with your finger and thumb and press until you hear a snapping sound. The two syringes will be aligned. Do not bend the pre-connected syringe system. |
|
|
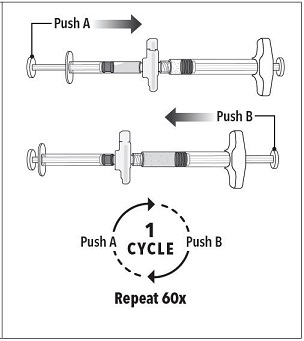
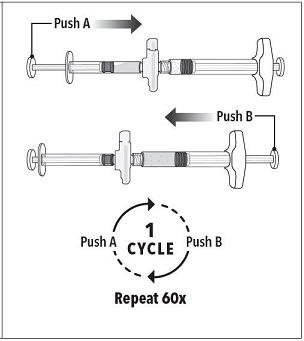
Step 3 Holding the syringes in a horizontal position, transfer the liquid contents of Syringe A into the leuprolide acetate powder contained in Syringe B. Thoroughly mix the product for 60 cycles by pushing the contents back and forth between both syringes to obtain a uniform suspension. *A cycle is one push of the Syringe A plunger and one push of the Syringe B plunger.
**Note:**Product must be mixed as described; shaking will NOT provide adequate mixing. Do not bend. |
|
|
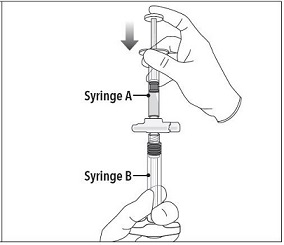
Step 4 After mixing, hold the syringes vertically (upright) with Syringe B (wide syringe) on the bottom. The syringes should remain securely coupled. Transfer all of the mixed product into Syringe B by depressing the Syringe A plunger and slightly withdrawing the Syringe B plunger. |
|
|
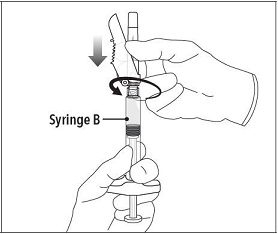
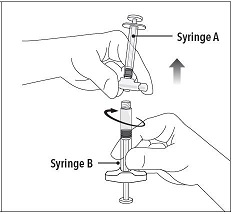
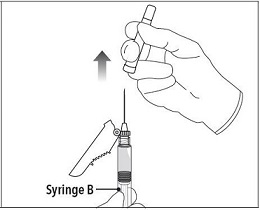
Step 5 While ensuring the Syringe A plunger is fully pushed down, hold the coupling device and unscrew Syringe B. This will disconnect Syringe B from the coupling device. Syringe A will remain attached to the coupling device. **Note:**Small air bubbles will remain in the formulation – this is acceptable. Do not purge the air bubbles from Syringe B as product may be lost! |
|
|
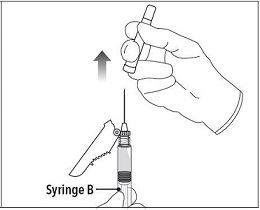
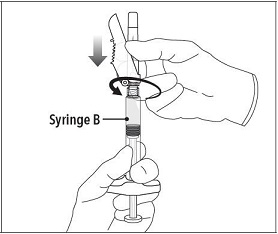
Step 6 Continue to hold Syringe B upright with the open end at the top. Hold back the white plunger on Syringe B to prevent loss of the product and attach the safety needle and cap. Gently screw clockwise with approximately a three- quarter turn until the safety needle and cap are secure. **Do not overtighten, as the needle hub may become damaged which could result in leakage of the product during injection.**The safety shield may also be damaged if the safety needle and cap are screwed with too much force. |
|
|
Step 7 Move the safety shield away from the needle and towards the syringe. Pull off the cap immediately prior to administration. |
|
Note: Should the needle hub appear to be damaged, or leak, do not use the product. If the needle hub is damaged or leakage is observed, use a new FENSOLVI carton.
2.4 Administration Instructions
|
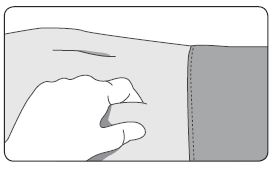
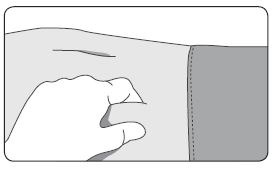
1. Select an injection site on the abdomen, upper buttocks, or another location with adequate amounts of subcutaneous tissue that does not have excessive pigment, nodules, lesions, or hair and hasn’t recently been used. 2. Cleanse the injection-site area with an alcohol swab (not enclosed). 3. Using the thumb and forefinger, grab and bunch the area of skin around the injection site. |
|
|
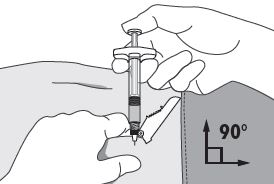
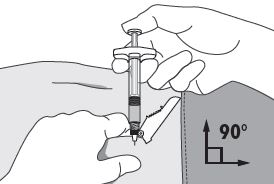
4. Using your dominant hand, insert the needle quickly at a 90° angle to the skin surface. The depth of penetration will depend on the amount and fullness of the subcutaneous tissue and the length of the needle. After the needle is inserted, release the skin. 5. Inject the drug using a slow, steady push and press down on the plunger until the syringe is empty. Make sure all the drug has been injected before removing the needle. 6. Withdraw the needle quickly at the same 90° angle used for insertion. |
|
|
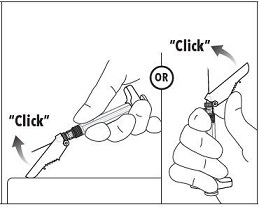
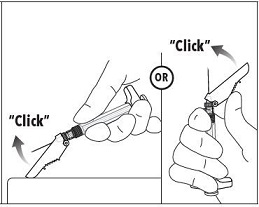
7. Immediately following the withdrawal of the needle, activate the safety shield using a finger/thumb or flat surface and push until it completely covers the needle tip and locks into place. 8. An audible and tactile “click” verifies a locked position. 9. Check to confirm the safety shield is fully engaged. Discard all components safely in an appropriate biohazard container. |
|
- Must be administered by a healthcare provider. (2.1)
- The dose of FENSOLVI is 45 mg administered by subcutaneous injection once every six months. (2.1)
- Monitor response to FENSOLVI with a GnRH agonist stimulation test, basal serum luteinizing hormone (LH) levels or serum concentration of sex steroid levels at 1 to 2 months following initiation of therapy and as needed to confirm adequate suppression of pituitary gonadotropins, sex steroids, and progression of secondary sexual characteristics. (2.2)
- Measure height every 3 to 6 months and monitor bone age periodically. (2.2)
- See Full Prescribing Information for reconstitution and administration instructions. (2.3, 2.4)
DESCRIPTION SECTION
11 DESCRIPTION
FENSOLVI for injectable suspension is a sterile polymeric matrix formulation of leuprolide acetate, a GnRH agonist, for subcutaneous use. It is designed to deliver leuprolide acetate at a controlled rate over a six-month therapeutic period.
Leuprolide acetate is a synthetic nonapeptide analog of naturally occurring gonadotropin releasing hormone. The analog possesses greater potency than the natural hormone. The chemical name is 5-oxo-L-prolyl-L-histidyl-L-tryptophyl- L-seryl-L-tyrosyl-D-leucyl-L-leucyl-L-arginyl-N-ethyl-L-prolinamide acetate (salt) with the following structural formula:
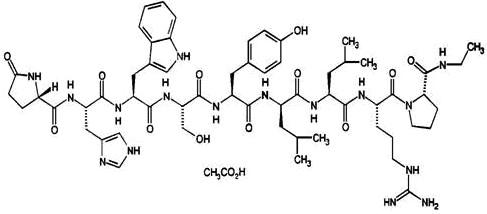
FENSOLVI is supplied as a pre-connected syringe system. Immediately prior to administration, the content of the pre-connected syringe system are mixed until homogenous. FENSOLVI is administered subcutaneously, where it forms a solid drug delivery depot.
Syringe A contains the in situ polymeric extended release technology consisting of a biodegradable poly (DL-lactide-co-glycolide) (PLG) polymer formulation dissolved in a biocompatible solvent, N-methyl-2pyrrolidone (NMP) and syringe B contains the leuprolide acetate.
Refer to Table 2 for the delivery system composition and reconstituted product formulation for FENSOLVI product.
Table 2: FENSOLVI Delivery System Composition and Reconstituted Product Formulation
|
In situ polymeric extended release technology |
Polymer |
PLG |
|
Polymer description |
Copolymer with hexanediol | |
|
Polymer DL-lactide to glycolide molar ratio |
85:15 | |
|
Reconstituted Product |
Polymer delivered |
165 mg |
|
NMP delivered |
165 mg | |
|
Leuprolide acetate delivered |
45 mg | |
|
Approximate leuprolide free base equivalent |
42 mg | |
|
Approximate administered formulation weight |
375 mg | |
|
Approximate injection volume |
0.375 mL |
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Leuprolide acetate, a GnRH agonist, acts as a potent inhibitor of gonadotropin secretion (LH and follicle stimulating hormone (FSH)) when given continuously in therapeutic doses. Following an initial stimulation of GnRH receptors, chronic administration of leuprolide acetate results in downregulation of GnRH receptors, reduction in release of LH, FSH and consequent suppression of ovarian and testicular production of estradiol and testosterone respectively. This inhibitory effect is reversible upon discontinuation of drug therapy.
12.2 Pharmacodynamics
In the clinical trial evaluating FENSOLVI in pediatric patients with CPP, there was a transient surge in circulating levels of LH, FSH, estradiol and testosterone following the first administration. A decrease in basal and GnRH agonist-stimulated LH and FSH levels along with reductions in basal estradiol and testosterone were observed after repeat administration.
12.3 Pharmacokinetics
Absorption
After an initial subcutaneous injection of FENSOLVI 45 mg in pediatric patients 4 to 9 years of age with CPP, leuprolide levels peaked 4 hours postdose with a mean Cmax of 212.3 ng/mL. Absorption occurred in two phases, a burst phase followed by a plateau phase. The mean plateau serum leuprolide level from 4 to 48 weeks was approximately 0.37 ng/mL with a range of 0.18 to 0.63 ng/mL. There was no accumulation of leuprolide after the second dose.
Distribution
The distribution of leuprolide following FENSOLVI administration was not evaluated in pediatric patients. The mean steady-state volume of distribution of leuprolide following intravenous bolus administration to healthy male volunteers was 27 L.
In vitro binding of leuprolide to human plasma proteins ranged from 43% to 49%.
Elimination
Metabolism
In rats and dogs, administration of 14C-labeled leuprolide was shown to be metabolized to smaller inactive peptides: a pentapeptide (Metabolite I), tripeptides (Metabolites II and III), and a dipeptide (Metabolite IV). These fragments may be further catabolized.
In healthy male volunteers, a 1 mg bolus of leuprolide administered intravenously revealed that the mean systemic clearance was 8.34 L/h, with a terminal elimination half-life of approximately 3 hours based on a two- compartment model.
Upon administration with different leuprolide acetate formulations, the major metabolite of leuprolide acetate is a pentapeptide (M-1) metabolite.
Specific Populations
The pharmacokinetics of FENSOLVI in hepatically and renally impaired pediatric patients have not been determined.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Two-year carcinogenicity studies were conducted with leuprolide acetate in rats and mice. In rats, a dose-related increase of benign pituitary hyperplasia and benign pituitary adenomas was noted at 24 months when the drug was administered subcutaneously at high daily doses (0.6 to 4 mg/kg). There was a significant but not dose-related increase of pancreatic islet-cell adenomas in females and of testicular interstitial cell adenomas in males (highest incidence in the low dose group). In mice, no leuprolide acetate- induced tumors or pituitary abnormalities were observed at a dose as high as 60 mg/kg for two years. Patients have been treated with leuprolide acetate for up to three years with doses as high as 10 mg/day and for two years with doses as high as 20 mg/day without demonstrable pituitary abnormalities. No carcinogenicity studies have been conducted with FENSOLVI.
Mutagenicity studies have been performed with leuprolide acetate using bacterial and mammalian systems. These studies provided no evidence of a mutagenic potential.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
The efficacy of FENSOLVI was evaluated in an uncontrolled, open-label, single arm clinical trial in which 64 pediatric patients (62 females and 2 males, naïve to previous GnRH agonist treatment) with CPP received at least one dose of FENSOLVI at a dosing interval of 24 weeks and were observed for 12 months. The mean age was 7.5 years (range 4 to 9 years) at the start of treatment. In pediatric patients with CPP, FENSOLVI reduced stimulated and basal gonadotropins to prepubertal levels. Suppression of peak stimulated LH concentrations to <4 IU/L was achieved in 87% of pediatric patients by month 6 and in 86% of patients by month 12. Suppression of estradiol or testosterone concentration to prepubertal levels at the 6-month assessment was achieved in 97% and 100% of patients, respectively. Suppression of estradiol or testosterone was maintained at the 12-month assessment with 98% (55/56 females) and 50% (1/2 males) maintaining suppression. FENSOLVI arrested or reversed progression of clinical signs of puberty with reductions in growth velocity and bone age. Mean growth velocity decreased from 8.9 ± 13.1 cm/yr at 1 month to 6.9 ± 3.1 cm/yr at 6 months and to 6.4 ± 1.9 cm/yr at 12 months.
Table 3: Reproductive Hormone Levels in Pediatric Patients with CPP Treated with FENSOLVI 45 mg Every 6 Months**********a******
|
% (n/N) of Patients Achieving Endpoints | ||||
|
Endpoint********b |
** Month 3** |
Month 6 |
Month 9 |
Month 12 |
|
LH levels < 4 IU/L |
85 (51/60) |
87 (54/62)c |
85 (50/59) |
86 (50/58) |
|
Estradiol levels < 73.4 pmol/L (< 20 pg/mL) |
98 (56/57) |
97 (58/60) |
98 (56/57) |
98 (55/56) |
|
Testosterone levels < 1 nmol/L (< 28.4 ng/dL) |
100 (2/2) |
100 (2/2) |
100 (2/2) |
50 (1/2) |
|
FSH levels < 2.5 IU/L |
62 (37/60) |
66 (41/62) |
44 (26/59) |
55(32/58) |
|
aIntent-to-treat Population (N=62) |
Eight female patients out of 62 did not meet the primary efficacy criteria for LH <4 IU/L at 6 months. In four of the eight patients, the LH level at 6 months was between 4.2 and 4.8 IU/L. The remaining four patients had LH levels
5 IU/L. However, post stimulation estradiol was suppressed to prepubertal levels (<20 pg/mL) in seven of the eight patients at month 6 and was maintained through month 12.
SPL UNCLASSIFIED SECTION
INSTRUCTIONS FOR USE
****Follow the detailed instructions below to ensure correct preparation of Fensolvi prior to administration:
|
Step 1 Use aseptic technique including gloves for reconstitution and administration.
Allow the product to reach room temperature before reconstitution to allow for
easier administration. Administer the product within 30 minutes or discard. | |
|
| |
|
Step 2 Grasp the latching button on the coupling device with your finger and thumb and press until you hear a snapping sound. The two syringes will be aligned. Do not bend the pre-connected syringe system. |
|
|
Step 3 Holding the syringes in a horizontal position, transfer the liquid contents of Syringe A into the leuprolide acetate powder contained in Syringe B. Thoroughly mix the product for 60 cycles by pushing the contents back and forth between both syringes to obtain a uniform suspension. *A cycle is one push of the Syringe A plunger and one push of the Syringe B plunger.
**Note:**Product must be mixed as described; shaking will NOT provide adequate mixing. Do not bend. |
|
|
Step 4 After mixing, hold the syringes vertically (upright) with Syringe B (wide syringe) on the bottom. The syringes should remain securely coupled. Transfer all of the mixed product into Syringe B by depressing the Syringe A plunger and slightly withdrawing the Syringe B plunger. |
|
|
Step 5 While ensuring the Syringe A plunger is fully pushed down, hold the coupling device and unscrew Syringe B. This will disconnect Syringe B from the coupling device. Syringe A will remain attached to the coupling device. **Note:**Small air bubbles will remain in the formulation – this is acceptable. Do not purge the air bubbles from Syringe B as product may be lost! |
|
|
Step 6 Continue to hold Syringe B upright with the open end at the top. Hold back the white plunger on Syringe B to prevent loss of the product and attach the safety needle and cap. Gently screw clockwise with approximately a three- quarter turn until the safety needle and cap are secure. **Do not overtighten, as the needle hub may become damaged which could result in leakage of the product during injection.**The safety shield may also be damaged if the safety needle and cap are screwed with too much force. |
|
|
Step 7 Move the safety shield away from the needle and towards the syringe. Pull off the cap immediately prior to administration. |
|
****Note: Should the needle hub appear to be damaged, or leak, do not use the product. If the needle hub is damaged or leakage is observed, use a new FENSOLVI carton.
|
1. Select an injection site on the abdomen, upper buttocks, or another location with adequate amounts of subcutaneous tissue that does not have excessive pigment, nodules, lesions, or hair and hasn’t recently been used. 2. Cleanse the injection-site area with an alcohol swab (not enclosed). 3. Using the thumb and forefinger, grab and bunch the area of skin around the injection site. |
|
|
4. Using your dominant hand, insert the needle quickly at a 90° angle to the skin surface. The depth of penetration will depend on the amount and fullness of the subcutaneous tissue and the length of the needle. After the needle is inserted, release the skin. 5. Inject the drug using a slow, steady push and press down on the plunger until the syringe is empty. Make sure all the drug has been injected before removing the needle. 6. Withdraw the needle quickly at the same 90° angle used for insertion. |
|
|
7. Immediately following the withdrawal of the needle, activate the safety shield using a finger/thumb or flat surface and push until it completely covers the needle tip and locks into place. 8. An audible and tactile “click” verifies a locked position. 9. Check to confirm the safety shield is fully engaged. Discard all components safely in an appropriate biohazard container. |
|
©2022 Tolmar
Manufactured by: Tolmar, Fort Collins, CO 80526 04006226 Rev. 1 04/23