IMJUDO
These highlights do not include all the information needed to use IMJUDO safely and effectively. See full prescribing information for IMJUDO.IMJUDO (tremelimumab-actl) injection, for intravenous useInitial U.S. Approval: 2022
6690679c-be2f-4588-a2e4-89fff74dd6be
HUMAN PRESCRIPTION DRUG LABEL
Jun 14, 2023
AstraZeneca Pharmaceuticals LP
DUNS: 054743190
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
tremelimumab
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
tremelimumab
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC 0310-4505-25Rx only
IMJUDO**®**25 mg/1.25 mL
(tremelimumab-actl) (20 mg/mL)
Injection
For Intravenous Infusion After Dilution
Single-dose vial. Discard unused portion.
Attention Pharmacist:
Dispense the accompanying Medication Guide
to each patient.
AstraZeneca

INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
1.1 Hepatocellular Carcinoma
IMJUDO, in combination with durvalumab, is indicated for the treatment of adult patients with unresectable hepatocellular carcinoma (uHCC).
1.2 Non-Small Cell Lung Cancer (NSCLC)
IMJUDO, in combination with durvalumab and platinum-based chemotherapy, is indicated for the treatment of adult patients with metastatic NSCLC with no sensitizing epidermal growth factor receptor (EGFR) mutations or anaplastic lymphoma kinase (ALK) genomic tumor aberrations.
IMJUDO is a cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) blocking antibody indicated:
•
in combination with durvalumab, for the treatment of adult patients with unresectable hepatocellular carcinoma (uHCC). (1.1)
•
in combination with durvalumab and platinum-based chemotherapy for the treatment of adult patients with metastatic non-small cell lung cancer (NSCLC) with no sensitizing epidermal growth factor receptor (EGFR) mutation or anaplastic lymphoma kinase (ALK) genomic tumor aberrations. (1.2)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
None.
None. (4)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling.
•
Immune-Mediated Adverse Reactions [see Warnings and Precautions (5.1)].
•
Infusion-Related Reactions [see Warnings and Precautions (5.2)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described in the Warnings and Precautions reflect exposure to IMJUDO 300 mg in combination with durvalumab 1,500 mg in 388 patients in HIMALAYA. In the HIMALAYA study patients received IMJUDO 300 mg administered as a single intravenous infusion in combination with durvalumab 1,500 mg on the same day, followed by durvalumab every 4 weeks.
The data also reflects exposure to IMJUDO 75 mg in combination with durvalumab 1,500 mg and histology-based platinum chemotherapy regimens in the pooled safety population (N=596) of 330 patients in POSEIDON [see Clinical Studies (14.1)], and 266 patients in CASPIAN who received up to four cycles of platinum-etoposide plus durvalumab 1,500 mg with tremelimumab-actl 75 mg every 3 weeks, followed by durvalumab 1,500 mg every 4 weeks (an unapproved regimen for extensive-stage small cell lung cancer). Of these patients, 64% received the maximum of 5 doses of IMJUDO and 79% received at least 4 doses.
In this pooled safety population, the most common (> 20%) adverse reactions were nausea (37%), decreased appetite (25%), and fatigue (22%). In this pooled safety population, the most common Grade 3 or 4 (> 10%) laboratory abnormalities were neutropenia (39%), leukopenia (21%), lymphocytopenia (20%), anemia (20%), hyponatremia (14%), lipase increased (12%), and thrombocytopenia (11%).
The data described in this section reflect exposure to IMJUDO in patients with uHCC included in the HIMALAYA study and in patients with metastatic NSCLC enrolled in the POSEIDON study.
Hepatocellular Carcinoma
Unresectable HCC - HIMALAYA
The safety of IMJUDO administered in combination with durvalumab was evaluated in a total of 388 patients with uHCC in HIMALAYA, a randomized, open-label, multicenter study [see Clinical Studies (14.1)]. Patients received IMJUDO 300 mg administered as a single intravenous infusion in combination with durvalumab 1,500 mg on the same day, followed by durvalumab every 4 weeks or sorafenib 400 mg given orally twice daily.
Serious adverse reactions occurred in 41% of patients who received IMJUDO in combination with durvalumab. Serious adverse reactions in > 1% of patients included hemorrhage (6%), diarrhea (4%), sepsis (2.1%), pneumonia (2.1%), rash (1.5%), vomiting (1.3%), acute kidney injury (1.3%), and anemia (1.3%). Fatal adverse reactions occurred in 8% of patients who received IMJUDO in combination with durvalumab, including death (1%), hemorrhage intracranial (0.5%), cardiac arrest (0.5%), pneumonitis (0.5%), hepatic failure (0.5%), and immune-mediated hepatitis (0.5%). The most common adverse reactions (occurring in ≥ 20% of patients) were rash, diarrhea, fatigue, pruritus, musculoskeletal pain, and abdominal pain.
Permanent discontinuation of the treatment regimen due to an adverse reaction occurred in 14% of patients; the most common adverse reactions leading to treatment discontinuation (≥ 1%) were hemorrhage (1.8%), diarrhea (1.5%), AST increased (1%), and hepatitis (1%).
Dosage interruptions or delay of the treatment regimen due to an adverse reaction occurred in 35% of patients. Adverse reactions which required dosage interruption or delay in ≥ 1% of patients included ALT increased (3.6%), diarrhea (3.6%), rash (3.6%), amylase increased (3.4%), AST increased (3.1%), lipase increased (2.8%), pneumonia (1.5%), hepatitis (1.5%), pyrexia (1.5%), anemia (1.3%), thrombocytopenia (1%), hyperthyroidism (1%), pneumonitis (1%), and blood creatinine increased (1%).
Table 5 summarizes the adverse reactions that occurred in patients treated with IMJUDO in combination with durvalumab in the HIMALAYA study.
Table 5. Adverse Reactions Occurring in ≥ 10% Patients in the HIMALAYA study|
IMJUDO and Durvalumab |
Sorafenib | |||
|---|---|---|---|---|
|
Adverse Reaction |
All Grades (%) |
Grade 3-4 (%) |
All Grades (%) |
Grade 3-4 (%) |
| ||||
|
Gastrointestinal disorders | ||||
|
Diarrhea* |
27 |
6 |
45 |
4.3 |
|
Abdominal pain* |
20 |
1.8 |
24 |
4 |
|
Nausea |
12 |
0 |
14 |
0 |
|
Skin and subcutaneous tissue disorders | ||||
|
Rash* |
32 |
2.8 |
57 |
12 |
|
Pruritus |
23 |
0 |
6 |
0.3 |
|
Metabolism and nutrition disorders | ||||
|
Decreased appetite |
17 |
1.3 |
18 |
0.8 |
|
General disorders and administration site conditions | ||||
|
Fatigue* |
26 |
3.9 |
30 |
6 |
|
Pyrexia* |
13 |
0.3 |
9 |
0.3 |
|
Psychiatric disorders | ||||
|
Insomnia |
10 |
0.3 |
4.3 |
0 |
|
Endocrine disorders | ||||
|
Hypothyroidism* |
14 |
0 |
6 |
0 |
|
Musculoskeletal and Connective Tissue Disorders | ||||
|
Musculoskeletal pain* |
22 |
2.6 |
17 |
0.8 |
Table 6 summarizes the laboratory abnormalities that occurred in patients treated with IMJUDO in combination with durvalumab in the HIMALAYA study.
Table 6. Laboratory Abnormalities Worsening from Baseline Occurring in ≥ 20% of Patients in the HIMALAYA study|
IMJUDO and Durvalumab |
Sorafenib | |||
|---|---|---|---|---|
|
Laboratory Abnormality |
Any grade* |
Grade 3*** or 4** |
Any grade* |
Grade 3*** or 4** |
| ||||
|
Chemistry | ||||
|
Aspartate Aminotransferase increased |
63 |
27 |
55 |
21 |
|
Alanine Aminotransferase increased |
56 |
18 |
53 |
12 |
|
Sodium decreased |
46 |
15 |
40 |
11 |
|
Bilirubin increased |
41 |
8 |
47 |
11 |
|
Alkaline Phosphatase increased |
41 |
8 |
44 |
5 |
|
Glucose increased |
39 |
14 |
29 |
4 |
|
Calcium decreased |
34 |
0 |
43 |
0.3 |
|
Albumin decreased |
31 |
0.5 |
37 |
1.7 |
|
Potassium increased |
28 |
3.8 |
21 |
2.6 |
|
Creatinine increased |
21 |
1.3 |
15 |
0.9 |
|
Hematology | ||||
|
Hemoglobin decreased |
52 |
4.8 |
40 |
6 |
|
Lymphocytes decreased |
41 |
11 |
39 |
10 |
|
Platelets decreased |
29 |
1.6 |
35 |
3.1 |
|
Leukocytes decreased |
20 |
0.8 |
30 |
1.1 |
Non-Small Cell Lung Cancer
Metastatic NSCLC – POSEIDON
The safety of IMJUDO in combination with durvalumab and platinum-based chemotherapy in patients with metastatic NSCLC was evaluated in POSEIDON (NCT03164616), a randomized, open-label, multicenter, active-controlled trial. A total of 330 patients received IMJUDO (≥ 30 kg body weight received 75 mg and ≤ 30kg body weight received 1mg/kg) in combination with durvalumab 1,500 mg and histology-based platinum chemotherapy regimens [see Clinical Studies (14.2)]. Of these patients, 66% received up to the maximum 5 doses of IMJUDO and 79% received at least 4 doses. Treatment was continued with durvalumab as a single agent (or with durvalumab and histology-based pemetrexed for non- squamous patients, based on the investigator’s decision) until disease progression or unacceptable toxicity. The trial excluded patients with active or prior autoimmune disease or with medical conditions that required systemic corticosteroids or immunosuppressants [see Clinical Studies (14.2)].
The median age of patients who received IMJUDO in combination with durvalumab and platinum-based chemotherapy was 63 years (range: 27 to 87); 80% male; 61% White, 29% Asian, 58% former smoker, 25% current smoker, and 68% ECOG performance of 1.
Serious adverse reactions occurred in 44% of patients receiving IMJUDO in combination with durvalumab and platinum-based chemotherapy. The most frequent serious adverse reactions reported in at least 2% of patients were pneumonia (11%), anemia (5%), diarrhea (2.4%), thrombocytopenia (2.4%), pyrexia (2.4%), and febrile neutropenia (2.1%). Fatal adverse reactions occurred in a total of 4.2% of patients receiving IMJUDO in combination with durvalumab and platinum- based chemotherapy. These include hepatitis, nephritis, myocarditis, pancreatitis (all in the same patient), death (2 patients), sepsis (2 patients), pneumonitis (2 patients), acute kidney injury (2 patients), febrile neutropenia (1 patient), chronic obstructive pulmonary disease (1 patient), dyspnea (1 patient), sudden death (1 patient), and ischemic stroke (1 patient).
Permanent discontinuation of IMJUDO or durvalumab due to an adverse reaction occurred in 17% of the patients. Adverse reactions which resulted in permanent discontinuation of IMJUDO or durvalumab in > 2% of patients included pneumonia.
Dosage interruptions or delay of IMJUDO and durvalumab due to an adverse reaction occurred in 41% of patients. Adverse reactions which required dosage interruption or delay of IMJUDO and durvalumab in > 1% of patients included anemia, leukopenia/white blood cell count decreased, pneumonia, pneumonitis, colitis, diarrhea, hepatitis, rash, asthenia, amylase increased, alanine aminotransferase increased, aspartate aminotransferase increased, lipase increased, neutropenia/neutrophil count decreased, and thrombocytopenia/platelet count decreased.
The most common adverse reactions (occurring in ≥ 20% of patients) were nausea, fatigue, musculoskeletal pain, decreased appetite, rash, and diarrhea. Grade 3 or 4 laboratory abnormalities (≥ 10%) were neutropenia, anemia, leukopenia, lymphocytopenia, lipase increased, hyponatremia and thrombocytopenia.
Table 7 summarizes the adverse reactions in POSEIDON.
Table 7. Adverse Reactions (≥ 10%) in Patients with NSCLC Who Received IMJUDO in the POSEIDON Study|
IMJUDO with durvalumab and platinum-based chemotherapy |
Platinum-based chemotherapy | |||
|---|---|---|---|---|
|
Adverse Reaction |
All Grades (%) |
Grade 3 or 4 (%) |
All Grades (%) |
Grade 3 or 4 (%) |
Þ ß à è ð | ||||
|
Respiratory, thoracic and mediastinal disorders | ||||
|
Cough/Productive Cough* |
12 |
0 |
8 |
0.3 |
|
Gastrointestinal disorders | ||||
|
Nausea |
42 |
1.8 |
37 |
2.1 |
|
Diarrhea |
22 |
1.5 |
15 |
1.5 |
|
Constipation |
19 |
0 |
24 |
0.6 |
|
Vomiting |
18 |
1.2 |
14 |
1.5 |
|
Stomatitis† |
10 |
0 |
6 |
0.3 |
|
Endocrine disorders | ||||
|
Hypothyroidism‡ |
13 |
0 |
2.1 |
0 |
|
Skin and subcutaneous tissue disorders | ||||
|
Rash§ |
27 |
2.4 |
10 |
0.6 |
|
Alopecia |
10 |
0 |
6 |
0 |
|
Pruritus |
11 |
0 |
4.5 |
0 |
|
General disorders and administration site conditions | ||||
|
Fatigue/Asthenia¶ |
36 |
5 |
32 |
4.5 |
|
Pyrexia# |
19 |
0 |
8 |
0 |
|
EdemaÞ |
10 |
0 |
10 |
0.6 |
|
Musculoskeletal and connective tissue disorders | ||||
|
Musculoskeletal Painß |
29 |
0.6 |
22 |
1.5 |
|
Metabolism and nutrition disorders | ||||
|
Decreased appetite |
28 |
1.5 |
25 |
1.2 |
|
Infections and Infestations | ||||
|
Pneumoniaà |
17 |
8 |
12 |
4.2 |
|
Upper respiratory tract infectionsè |
15 |
0.6 |
9 |
0.9 |
|
Nervous system disorders | ||||
|
Headacheð |
11 |
0 |
8 |
0.6 |
Table 8 summarizes the laboratory abnormalities in POSEIDON.
Table 8: Select Laboratory Abnormalities (≥ 10%) That Worsened from Baseline in Patients with NSCLC Who Received IMJUDO in the POSEIDON Study|
Laboratory Abnormality* |
IMJUDO with Durvalumab and Platinum-based chemotherapy† |
Platinum-based chemotherapy‡ | ||
|---|---|---|---|---|
|
All Grades |
Grade 3 or 4 |
All Grades |
Grade 3 or 4 | |
| ||||
|
Chemistry | ||||
|
Lipase increased |
35 |
14 |
25 |
5 |
|
Hyponatremia |
55 |
13 |
50 |
11 |
|
Hypernatremia |
15 |
0 |
14 |
0 |
|
Amylase increased |
41 |
9 |
25 |
6 |
|
Hypokalemia |
21 |
7 |
17 |
2.8 |
|
Hyperglycemia |
42 |
6 |
37 |
3.1 |
|
Increased ALT |
64 |
6 |
56 |
4.7 |
|
Increased AST |
63 |
5 |
55 |
2.2 |
|
Blood creatinine increased |
89 |
4.0 |
83 |
1.9 |
|
Increased Alkaline Phosphatase |
33 |
3.4 |
26 |
1.2 |
|
Gamma Glutamyl Transferase increased |
38 |
2.2 |
35 |
4.7 |
|
Hyperkalemia |
49 |
2.2 |
35 |
2.8 |
|
Albumin decreased |
27 |
1.9 |
18 |
0.9 |
|
Hypocalcemia |
58 |
0.9 |
49 |
0.9 |
|
Hypomagnesemia |
12 |
4 |
23 |
0 |
|
Bilirubinemia |
16 |
0.9 |
8 |
0.3 |
|
Hematology | ||||
|
Neutropenia |
71 |
37 |
69 |
32 |
|
Anemia |
84 |
24 |
84 |
25 |
|
Leukopenia |
77 |
21 |
81 |
18 |
|
Lymphocytopenia |
67 |
20 |
60 |
19 |
|
Thrombocytopenia |
53 |
11 |
54 |
12 |
Most common adverse reactions (≥ 20%) of patients with uHCC are rash, diarrhea, fatigue, pruritus, musculoskeletal pain, and abdominal pain. Most common laboratory abnormalities (≥ 40%) of patients with uHCC are AST increased, ALT increased, hemoglobin decreased, sodium decreased, bilirubin increased, alkaline phosphatase increased, and lymphocytes decreased. (6.1)
Most common adverse reactions (≥ 20%) of patients with metastatic NSCLC were nausea, fatigue, musculoskeletal pain, decreased appetite, rash, and diarrhea. (6.1)
**To report SUSPECTED ADVERSE REACTIONS, contactAstraZeneca at 1-800-236-9933 or FDA at 1-800-FDA-1088 or **www.fda.gov/medwatch.
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
Injection: 25 mg/1.25 mL (20 mg/mL) or 300 mg/15 mL (20 mg/mL) clear to slightly opalescent, colorless to slightly yellow solution in a single-dose vial.
•
Injection: 25 mg/1.25 mL (20 mg/mL) solution in a single-dose vial. (3)
•
Injection: 300 mg/15 mL (20 mg/mL) solution in a single-dose vial. (3)
RECENT MAJOR CHANGES SECTION
RECENT MAJOR CHANGES
Indications and Usage (1.2) 11/2022
Dosage and Administration (2.1, 2.3) 11/2022
Dosage and Administration (2.3) 06/2023
Warnings and Precautions (5.1, 5.2) 11/2022
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk summary
Based on findings from animal studies and its mechanism of action, IMJUDO can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. There are no available data on the use of IMJUDO in pregnant women. In animal studies, CTLA-4 blockade is associated with increased risk of immune-mediated rejection of the developing fetus and fetal death (see Data).
Human immunoglobulin G2 (IgG2) is known to cross the placental barrier; therefore, IMJUDO has the potential to be transmitted from the mother to the developing fetus. Advise pregnant women and females of reproductive potential of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
In a reproduction study, administration of tremelimumab-actl to pregnant cynomolgus monkeys during the period of organogenesis was not associated with maternal toxicity or effects on embryo-fetal development at exposure levels approximately 4 to 31-times higher than those observed at a recommended dose range of 75 mg to 300 mg based on area under the curve (AUC). CTLA-4 plays a role in maintaining maternal immune tolerance to the fetus to preserve pregnancy and in immune regulation of the newborn. In a murine model of pregnancy, CTLA-4 blockade resulted in increased resorptions and reduced live fetuses. Mated genetically engineered mice heterozygous for CTLA-4 (CTLA-4+/-) gave birth to CTLA-4+/- offspring and offspring deficient in CTLA-4 (homozygous negative, CTLA-4-/-) that appeared healthy at birth. The CTLA-4-/- homozygous negative offspring developed signs of a lymphoproliferative disorder and died by 3 to 4 weeks of age with multiorgan tissue destruction. Based on its mechanism of action, fetal exposure to tremelimumab-actl may increase the risk of developing immune-mediated disorders or altering the normal immune response.
8.2 Lactation
Risk Summary
There are no data on the presence of tremelimumab-actl in human milk, its effects on a breastfed child, or on milk production. Maternal IgG is known to be present in human milk. The effects of local gastrointestinal exposure and limited systemic exposure in the breastfed child to IMJUDO are unknown. Because of the potential for serious adverse reactions in the breastfed child, advise women not to breastfeed during treatment with IMJUDO and for 3 months after the last dose. Refer to the Prescribing Information for agents administered in combination with IMJUDO for breastfeeding recommendations, as appropriate.
8.3 Females and Males of Reproductive Potential
IMJUDO can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify pregnancy status of females of reproductive potential prior to initiating treatment with IMJUDO.
Contraception
Advise females of reproductive potential to use effective contraception during treatment with IMJUDO and for 3 months after the last dose. Refer to the Prescribing Information for the agents administered in combination with IMJUDO for recommended contraception duration, as appropriate.
8.4 Pediatric Use
The safety and effectiveness of tremelimumab-actl have not been established in pediatric patients.
8.5 Geriatric Use
Of the 393 patients with uHCC treated with IMJUDO in combination with durvalumab, 50% of patients were 65 years or older and 13% of patients were 75 years or older. No overall differences in safety or efficacy of IMJUDO have been observed between patients 65 years or older and younger adult patients.
Of the 330 patients with metastatic NSCLC treated with IMJUDO in combination with durvalumab and platinum-based chemotherapy, 143 (43%) patients were 65 years or older and 35 (11%) patients were 75 years or older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects.
Lactation: Advise not to breastfeed. (8.2)
DESCRIPTION SECTION
11 DESCRIPTION
Tremelimumab-actl, a cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) blocking human IgG2 monoclonal antibody, is produced by recombinant DNA technology in NS0 cell suspension culture and has a molecular weight of 149 kDa.
IMJUDO (tremelimumab-actl) injection is a sterile, preservative-free, clear to slightly opalescent, colorless to slightly yellow solution, in a single-dose vial for intravenous infusion after dilution. IMJUDO contains tremelimumab- actl at a concentration of 20 mg/mL in either a 25 mg/1.25 mL or a 300 mg/15 mL single-dose vial.
Each mL contains 20 mg of tremelimumab-actl, and edetate disodium (0.09 mg), histidine (0.68 mg), L‑histidine hydrochloride monohydrate (3.3 mg), polysorbate 80 (0.2 mg), trehalose (76 mg), and Water for Injection, USP. The pH is approximately 5.5.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
IMJUDO (tremelimumab-actl) injection is a clear to slightly opalescent, colorless to slightly yellow solution supplied in a carton containing one single-dose vial in the following concentrations:
•
25 mg/1.25 mL (20 mg/mL) (NDC 0310-4505-25)
•
300 mg/15 mL (20 mg/mL) (NDC 0310-4535-30)
Store in a refrigerator at 2°C to 8°C (36°F to 46°F) in original carton to protect from light.
Do not freeze. Do not shake.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
CTLA-4 is a negative regulator of T-cell activity. Tremelimumab--actl is a monoclonal antibody that binds to CTLA-4 and blocks the interaction with its ligands CD80 and CD86, releasing CTLA-4-mediated inhibition of T-cell activation. In synergistic mouse tumor models, blocking CTLA-4 activity resulted in decreased tumor growth and increased proliferation of T cells in tumors.
12.2 Pharmacodynamics
The exposure-response relationship and time course of pharmacodynamic response for the safety and effectiveness of tremelimumab-actl have not been fully characterized.
12.3 Pharmacokinetics
The pharmacokinetics of tremelimumab-actl was studied in patients with other solid tumors following administration of doses 1 mg/kg, 3 mg/kg, and 10 mg/kg (1- to 10-times the approved recommended dosage) administered once every 4 weeks for 4 doses. The pharmacokinetics of tremelimumab-actl as a single dose of 300 mg were evaluated in patients with HCC.
The AUC of tremelimumab-actl increased proportionally from 1 mg/kg to 10 mg/kg every 4 weeks (1 to 10-times the approved recommended dosage) and steady state was achieved at approximately 12 weeks.
Distribution
The geometric mean (% coefficient of variation [CV%]) of tremelimumab-actl for central (V1) and peripheral (V2) volume of distribution was 3.45 (24%) and 2.66 (34%) L, respectively.
Elimination
The geometric mean (CV%) terminal half-life of tremelimumab-actl was 16.9 days (19%) after a single dose and 18.2 days (19%) during steady state. The geometric mean (CV%) clearance of tremelimumab-actl was 0.286 L/day (32%) after a single dose and 0.263 L/day (32%) during steady state.
Specific Populations
There were no clinically significant differences in the pharmacokinetics of tremelimumab-actl based on body weight (34 to149 kg), age (18 to 87 years), sex, race (White, Black, Asian, Native Hawaiian, Pacific Islander, or American Indian), serum albumin levels (0.3 to 396 g/L), lactate dehydrogenase levels (12 to 5570 U/L), soluble PD-L1 (67 to 349 pg/mL), tumor type (NSCLC, HCC), organ dysfunction including mild to moderate renal impairment (CLcr 30 to 89 mL/min), and mild to moderate hepatic impairment (bilirubin < 3 x ULN and any AST).
The effect of severe renal impairment (CLcr 15 to 29 mL/min) or severe hepatic impairment (bilirubin > 3 x ULN and any AST) on the pharmacokinetics of tremelimumab-actl is unknown.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies (ADA) is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of ADA in the studies described below with the incidence of ADA in other studies, including those of tremelimumab-actl.
In the HIMALAYA study, of the 182 patients who were treated with a single dose of tremelimumab-actl in combination with durvalumab once in every 4 weeks therapy and evaluable for the presence of ADAs against tremelimumab-actl at predose week 0 and week 4, 11% (20/182) of patients tested positive for anti- tremelimumab-actl antibodies. Among the 20 patients who tested positive for ADAs 40% (8/20) tested positive for neutralizing antibodies against tremelimumab-actl. There was no identified clinically significant effect of anti-tremelimumab antibodies on the pharmacokinetics or safety of tremelimumab-actl; however, the effect of ADAs and neutralizing antibodies on the effectiveness of tremelimumab-actl is unknown.
In the POSEIDON study, of the 278 ADA-evaluable patients who were treated with IMJUDO 75 mg for up to five doses in combination with durvalumab 1,500 mg and platinum-based chemotherapy every 3 weeks and evaluated for presence of ADAs against tremelimumab-actl at pre-dose week 0, week 3, and week 12, 14% (38/278) of patients tested positive for anti-tremelimumab-actl antibodies. Among the 38 patients who tested positive for ADAs, 82% (31/38) tested positive for neutralizing antibodies against tremelimumab-actl. There was no identified clinically significant effect of anti-tremelimumab-actl antibodies on pharmacokinetics or safety of tremelimumab-actl, however, the effect of ADAs on effectiveness of tremelimumab-actl is unknown.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenic and genotoxic potential of tremelimumab-actl have not been evaluated.
Animal fertility studies have not been conducted with tremelimumab-actl.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Hepatocellular Carcinoma (HCC)
Unresectable HCC - HIMALAYA
The efficacy of IMJUDO in combination with durvalumab was evaluated in the HIMALAYA study (NCT03298451), a randomized (1:1:1), open-label, multicenter study in patients with confirmed uHCC who had not received prior systemic treatment for HCC. Patients were randomized to one of two investigational arms (IMJUDO plus durvalumab or durvalumab) or sorafenib. Study treatment consisted of IMJUDO as a one-time single intravenous infusion of 300 mg in combination with durvalumab 1,500 mg on the same day, followed by durvalumab every 4 weeks; durvalumab 1,500 mg every 4 weeks; or sorafenib 400 mg given orally twice daily, until disease progression or unacceptable toxicity. The efficacy assessment of IMJUDO is based on patients randomized to the IMJUDO plus durvalumab arm versus the sorafenib arm. Randomization was stratified by macrovascular invasion (MVI) (yes or no), etiology of liver disease (hepatitis B virus vs. hepatitis C virus vs. others) and ECOG performance status (0 vs. 1).
The study enrolled patients with BCLC Stage C or B (not eligible for locoregional therapy). The study excluded patients with co-infection of viral hepatitis B and hepatitis C; active or prior documented gastrointestinal (GI) bleeding within 12 months; ascites requiring non-pharmacologic intervention within 6 months; hepatic encephalopathy within 12 months before the start of treatment; active or prior documented autoimmune or inflammatory disorders. Esophagogastroduodenoscopy was not mandated prior to enrollment but adequate endoscopic therapy, according to institutional standards, was required for patients with a history of esophageal variceal bleeding or those assessed as high risk for esophageal variceal bleeding by the treating physician.
Study treatment was permitted beyond disease progression if the patient was clinically stable and was deriving clinical benefit as determined by the investigator.
The major efficacy outcome measure was overall survival (OS) between the IMJUDO plus durvalumab arm versus the sorafenib arm. Additional efficacy outcomes were investigator-assessed progression-free survival (PFS), objective response rate (ORR) and duration of response (DoR) according to RECIST v1.1. Tumor assessments were conducted every 8 weeks for the first 12 months and then every 12 weeks thereafter.
The baseline demographics of the IMJUDO plus durvalumab and sorafenib arms were as follows: male (85%), age < 65 years (50%), median age of 65 years (range: 18 to 88 years), White (46%), Asian (49%), Black or African American (2%), Native Hawaiian or other Pacific Islander (0.1%), race Unknown (2%), Hispanic or Latino (5%), Not Hispanic or Latino (94%), ethnicity Unknown (1%), ECOG PS 0 (62%); Child-Pugh Class score A (99%), macrovascular invasion (26%), extrahepatic spread (53%), viral etiology hepatitis B (31%), hepatitis C (27%), uninfected (42%).
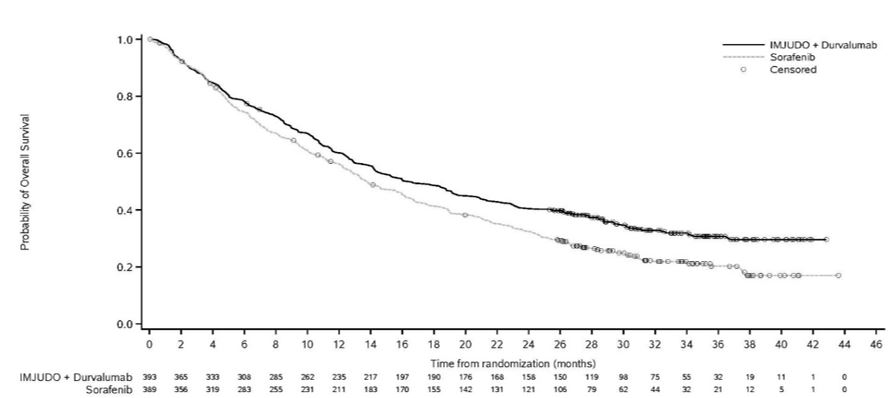
Efficacy results are presented in Table 9 and Figure 1.
Table 9. Efficacy Results for HIMALAYA Study|
Endpoint |
IMJUDO and Durvalumab |
Sorafenib |
|---|---|---|
| ||
|
OS | ||
|
Number of deaths (%) |
262 (66.7) |
293 (75.3) |
|
16.4 (14.2, 19.6) |
13.8 (12.3, 16.1) |
|
0.78 (0.66, 0.92) | |
|
0.0035 | |
|
PFS | ||
|
Number of events (%) |
335 (85.2) |
327 (84.1) |
|
Median PFS (months) (95% CI) |
3.8 (3.7, 5.3) |
4.1 (3.7, 5.5) |
|
HR (95% CI)* |
0.90 (0.77, 1.05) | |
|
ORR | ||
|
20.1 (16.3, 24.4) |
5.1 (3.2, 7.8) |
|
12 (3.1) |
0 |
|
67 (17.0) |
20 (5.1) |
|
DoR | ||
|
22.3 (13.7, NR) |
18.4 (6.5, 26.0) |
|
82.3 |
78.9 |
|
65.8 |
63.2 |
|
CI=Confidence Interval, HR=Hazard Ratio, NR=Not Reached |
Figure 1. Kaplan-Meier curve of OS
****
14.2 Metastatic NSCLC
Metastatic NSCLC – POSEIDON
The efficacy of IMJUDO in combination with durvalumab and platinum-based chemotherapy in previously untreated metastatic NSCLC patients with no sensitizing epidermal growth factor receptor (EGFR) mutation or anaplastic lymphoma kinase (ALK) genomic tumor aberrations was investigated in POSEIDON, a randomized, multicenter, active-controlled, open-label trial (NCT03164616). Eligible patients had Eastern Cooperative Oncology Group (ECOG) Performance Status of 0 or 1 and must have had no prior chemotherapy or any other systemic therapy for metastatic NSCLC. Choice of platinum-based chemotherapy was at the Investigator’s discretion, taking into consideration the calculated creatinine clearance. Patients with active and/or untreated brain metastases; a history of active primary immunodeficiency; autoimmune disorders including active or prior documented autoimmune or inflammatory disorders; use of systemic immunosuppressants within 14 days before the first dose of the treatment except physiological dose of systemic corticosteroids were ineligible.
Randomization was stratified by tumor cells (TC) PD-L1 expression (TC ≥ 50% vs. TC < 50%), disease stage (Stage IVA vs. Stage IVB), and histology (non- squamous vs. squamous).
Patients were randomized 1:1:1 to receive IMJUDO in combination with durvalumab and platinum-based chemotherapy according to the regimens listed below, durvalumab and platinum-based chemotherapy (an unapproved regimen for metastatic NSCLC), or platinum-based chemotherapy. The evaluation of efficacy for metastatic NSCLC relied on comparison between:
•
IMJUDO 75 mg (or 1mg/kg for patients < 30kg) with durvalumab 1,500 mg and platinum-based chemotherapy every 3 weeks for 4 cycles, followed by durvalumab 1,500 mg every 4 weeks as a single agent. A fifth dose of IMJUDO 75 mg (or 1mg/kg for patients < 30kg) was given at Week 16 in combination with durvalumab dose 6.
•
Platinum-based chemotherapy every 3 weeks as monotherapy for 4 cycles. Patients could receive an additional 2 cycles (a total of 6 cycles post-randomization), as clinically indicated, at Investigator’s discretion.
Patients received IMJUDO and durvalumab in combination with one of the following platinum-based chemotherapy regimens:
•
Non-squamous NSCLC
•
Pemetrexed 500 mg/m2 with carboplatin AUC 5-6 or cisplatin 75 mg/m2 every 3 weeks for 4 cycles
•
Squamous NSCLC
•
Gemcitabine 1,000 or 1,250 mg/m2 on Days 1 and 8 with cisplatin 75 mg/m2 or carboplatin AUC 5-6 on Day 1 every 3 weeks for 4 cycles
•
Non-squamous and Squamous NSCLC
•
Nab-paclitaxel 100 mg/m2 on Days 1, 8, and 15 with carboplatin AUC 5-6 on Day 1 every 3 weeks for 4 cycles
IMJUDO was given up to a maximum of 5 doses. Durvalumab and histology-based pemetrexed continued every 4 weeks until disease progression or unacceptable toxicity. Administration of durvalumab monotherapy was permitted beyond disease progression if the patient was clinically stable and deriving clinical benefit as determined by the Investigator. Patients with disease progression during durvalumab monotherapy were given the option to be retreated with 4 additional cycles of IMJUDO in combination with durvalumab. Tumor assessments were performed at Week 6, Week 12, and then every 8 weeks thereafter.
The major efficacy outcome measures were progression free survival (PFS) and overall survival (OS) of IMJUDO and durvalumab in combination with platinum- based chemotherapy compared to platinum-based chemotherapy alone. Additional efficacy outcome measures were overall response rate (ORR) and duration of response (DoR). PFS, ORR, and DoR were assessed using Blinded Independent Central Review (BICR) according to RECIST v1.1.
A total of 675 patients were randomized to receive either IMJUDO with durvalumab and platinum-based chemotherapy (n=338) or platinum-based chemotherapy (n=337). The median age was 63 years (range: 27 to 87), 46% of patients age ≥ 65 years, 77% male, 57% White, 34% Asian, 0.3% Native Hawaiian or Other Pacific Islander, 3% American Indian or Alaska Native, 2% Black or African American, 4% Other Race, 79% former or current smoker, 34% ECOG PS 0, and 66% ECOG PS 1. Thirty-six percent had squamous histology, 63% non-squamous histology, 29% PD-L1 expression TC ≥ 50%, 71% PD-L1 expression TC < 50%.
Efficacy results are summarized in Table 10 and Figure 2.
Table 10. Efficacy Results for POSEIDON|
IMJUDO with durvalumab and platinum-based chemotherapy (n=338) |
Platinum-based chemotherapy | |
|---|---|---|
| ||
|
OS* | ||
|
251 (74) |
285 (85) |
|
14.0 (11.7, 16.1) |
11.7 (10.5, 13.1) |
|
0.77 (0.65, 0.92) | |
|
0.00304 | |
|
PFS* | ||
|
238 (70) |
258 (77) |
|
6.2 (5.0, 6.5) |
4.8 (4.6, 5.8) |
|
0.72 (0.60, 0.86) | |
|
0.00031 | |
|
ORR % (95% CI)‡ |
39 (34, 44) |
24 (20, 29) |
|
Median DoR (months) |
9.5 (7.2, NR) |
5.1 (4.4, 6.0) |
|
NR=Not Reached, CI=Confidence Interval |
Figure 2. Kaplan-Meier curves of OS in POSEIDON
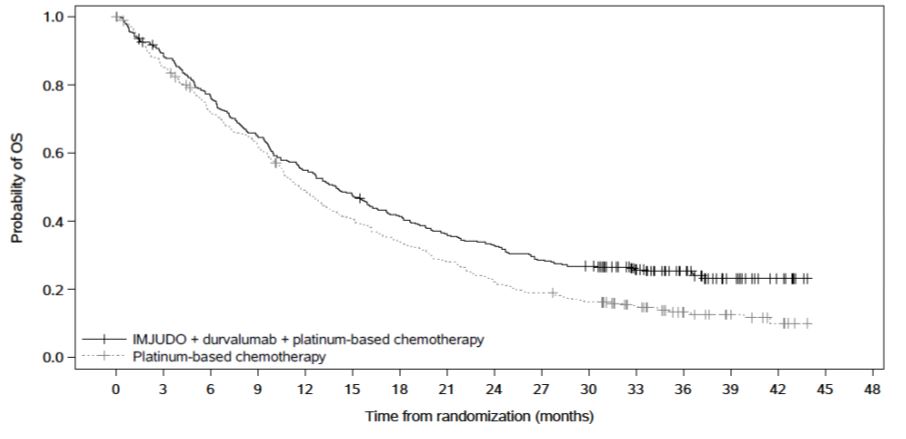
|
Number of patients at risk | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Month |
0 |
3 |
6 |
9 |
12 |
15 |
18 |
21 |
24 |
27 |
30 |
33 |
36 |
39 |
42 |
45 |
|
IMJUDO + durvalumab + platinum-based chemotherapy | ||||||||||||||||
|
338 |
298 |
256 |
217 |
183 |
159 |
137 |
120 |
109 |
95 |
88 |
64 |
41 |
20 |
9 |
0 | |
|
Platinum-based chemotherapy | ||||||||||||||||
|
337 |
284 |
236 |
204 |
160 |
132 |
111 |
91 |
72 |
62 |
52 |
38 |
21 |
13 |
6 |
0 |
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Immune-Mediated Adverse Reactions
Inform patients of the risk of immune-mediated adverse reactions that may require corticosteroid treatment and interruption or discontinuation of IMJUDO in combination with durvalumab, including [see Warnings and Precautions (5.1)]:
•
Pneumonitis: Advise patients to contact their healthcare provider immediately for any new or worsening cough, chest pain, or shortness of breath.
•
Colitis: Advise patients to contact their healthcare provider immediately for diarrhea, blood or mucus in stools, or severe abdominal pain.
•
Hepatitis: Advise patients to contact their healthcare provider immediately for jaundice, severe nausea or vomiting, pain on the right side of abdomen, lethargy, or easy bruising or bleeding.
•
Endocrinopathies: Advise patients to contact their healthcare provider immediately for signs or symptoms of hypothyroidism, hyperthyroidism, adrenal insufficiency, type 1 diabetes mellitus, or hypophysitis.
•
Nephritis: Advise patients to contact their healthcare provider immediately for signs or symptoms of nephritis.
•
Dermatological Reactions: Advise patients to contact their healthcare provider immediately for signs or symptoms of severe dermatological reactions.
•
Pancreatitis: Advise patients to contact their healthcare provider immediately for signs or symptoms of pancreatitis.
•
Other Immune-Mediated Adverse Reactions: Advise patients to contact their healthcare provider immediately for signs or symptoms of aseptic meningitis, immune thrombocytopenia, myocarditis, hemolytic anemia, myositis, uveitis, keratitis, and myasthenia gravis.
Infusion-Related Reactions:
•
Advise patients to contact their healthcare provider immediately for signs or symptoms of infusion-related reactions [see Warnings and Precautions (5.2)].
Embryo-Fetal Toxicity:
•
Advise females of reproductive potential that IMJUDO can cause harm to a fetus and to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.3) and Use in Specific Populations (8.1, 8.3)].
•
Advise females of reproductive potential to use effective contraception during treatment and for 3 months after the last dose of IMJUDO [see Use in Specific Populations (8.3)].
Lactation:
•
Advise female patients not to breastfeed while taking IMJUDO and for 3 months after the last dose [see Warnings and Precautions (5.3) and Use in Specific Populations (8.2)].
Manufactured for:
AstraZeneca Pharmaceuticals LP
Wilmington, DE 19850
Manufactured By:
AstraZeneca AB
Södertälje, Sweden SE-15185
US License No. 2059
IMJUDO® is a registered trademark of AstraZeneca group of companies.
©AstraZeneca 2022
SPL MEDGUIDE SECTION
|
MEDICATION GUIDE (tremelimumab-actl) injection | ||
|
What is the most important information I should know about IMJUDO? IMJUDO is a medicine that may treat certain cancers by working with your immune system. IMJUDO in combination with durvalumab can cause your immune system to attack normal organs and tissues in any area of your body and can affect the way they work. These problems can sometimes become severe or life-threatening and can lead to death. You can have more than one of these problems at the same time. These problems may happen anytime during treatment or even after your treatment has ended. Call or see your healthcare provider right away if you develop any new or worsening signs or symptoms, including: Lung problems. | ||
|
• |
• |
• |
|
Intestinal problems. | ||
|
• • |
• | |
|
Liver problems. | ||
|
• • • |
• • | |
|
Hormone gland problems. | ||
|
• • • • • • • • |
• • • • • • • | |
|
Kidney problems. | ||
|
• • |
• • | |
|
Skin problems. | ||
|
• • • |
• • • | |
|
Pancreas problems. | ||
|
• • |
• | |
|
Problems can also happen in other organs and tissues. These are not all of the signs and symptoms of immune system problems that can happen with IMJUDO. Call or see your healthcare provider right away for any new or worsening signs or symptoms, which may include: • • • • • • | ||
|
Infusion reactions that can sometimes be severe or life-threatening. Signs and symptoms of infusion reactions may include: | ||
|
• • • • |
• • • • | |
|
Getting medical treatment right away may help keep these problems from becoming more serious. Your healthcare provider will check you for these problems during your treatment with IMJUDO. Your healthcare provider may treat you with corticosteroid or hormone replacement medicines. Your healthcare provider may also need to delay or completely stop treatment with IMJUDO, if you have severe side effects. | ||
|
What is IMJUDO? • • o o It is not known if IMJUDO is safe and effective in children. | ||
|
Before you receive IMJUDO, tell your healthcare provider about all of your medical conditions, including if you: • • • Females who are able to become pregnant o o o • Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. | ||
|
How will I receive IMJUDO? • • • o o o • o o o o • • | ||
|
What are the possible side effects of IMJUDO? IMJUDO can cause serious side effects, including: See “What is the most important information I should know about IMJUDO?” The most common side effects of IMJUDO when used in combination with durvalumab in adults with uHCC include: | ||
|
• • • |
• • • |
|
The most common side effects of IMJUDO when used in combination with durvalumab and platinum-containing chemotherapy in adults with metastatic NSCLC include: | |
|
• • • |
• • • |
|
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of IMJUDO. Ask your healthcare provider or pharmacist for more information. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
General information about the safe and effective use of IMJUDO. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. If you would like more information about IMJUDO, talk with your healthcare provider. You can ask your healthcare provider for information about IMJUDO that is written for health professionals. |
|
What are the ingredients in IMJUDO? **Active ingredient:**tremelimumab-actl **Inactive ingredients:**edetate disodium, histidine, L-histidine hydrochloride monohydrate, polysorbate 80, trehalose, and Water for Injection, USP. |
|
Manufactured for: AstraZeneca Pharmaceuticals LP, Wilmington, DE 19850 Manufactured by: AstraZeneca AB, Södertälje, Sweden SE-15185 US License No. 2059 IMJUDO® is a registered trademark of AstraZeneca group of companies. For more information, call 1-800-236-9933 or go to www.IMJUDO.com © AstraZeneca 2024 |
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 01/2024
