Super lightening Based on gold 24k and kojic acid Cream
36148dd7-a0e2-0143-e063-6394a90a5069
HUMAN OTC DRUG LABEL
May 26, 2025
Guangzhou Kadiya Biotechnology Co., Ltd.
DUNS: 713172913
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Super lightening Based on gold 24k and kojic acid Cream
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (27)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Active Ingredients
CARICA PAPAYA FRUIT EXTRACT
Uses:
For Facial Skin Moisturizing, Antioxidant, Blemish Removal, Whitening,
Brightening
The storage method:
Store sealed in a cool place and out of sunlight
Warning.
1.Before use, perform a skin test on your arm and discontinue use if you have
allergy symptoms or skin abnormalities.
2、For external use only, avoid contact with eyes when used
3、Keep out of reach of children
Inactive ingredients:
AQUA,MINERAL OIL,GLYCERIN,Polyethylene,PROPYLENE GLYCOL,STEARYL
ALCOHOL,BUTYLENE GLYCOL,Steareth-2,Steareth-21,CETYL
ALCOHOL,DIMETHICONE,Isosorbide Dimethyl Ether,GLYCERYL
STEARATE,PEG-100,PHENOXYETHANOL,ALLANTOIN,Polyacrylamide,ALOE BARBADENSIS LEAF
EXTRACT,ANTHEMIS NOBILIS FLOWER EXTRACT,PORTULACA OLERACEA FLOWER/LEAF/STEM
EXTRACT,PELARGONIUM GRAVEOLENS FLOWER/LEAF/STEM EXTRACT,XANTHAN
GUM,FRAGRANCE,ETHYLHEXYLGLYCERIN, C13-14 Isoparaffin,DISODIUM EDTA,Laureth-7
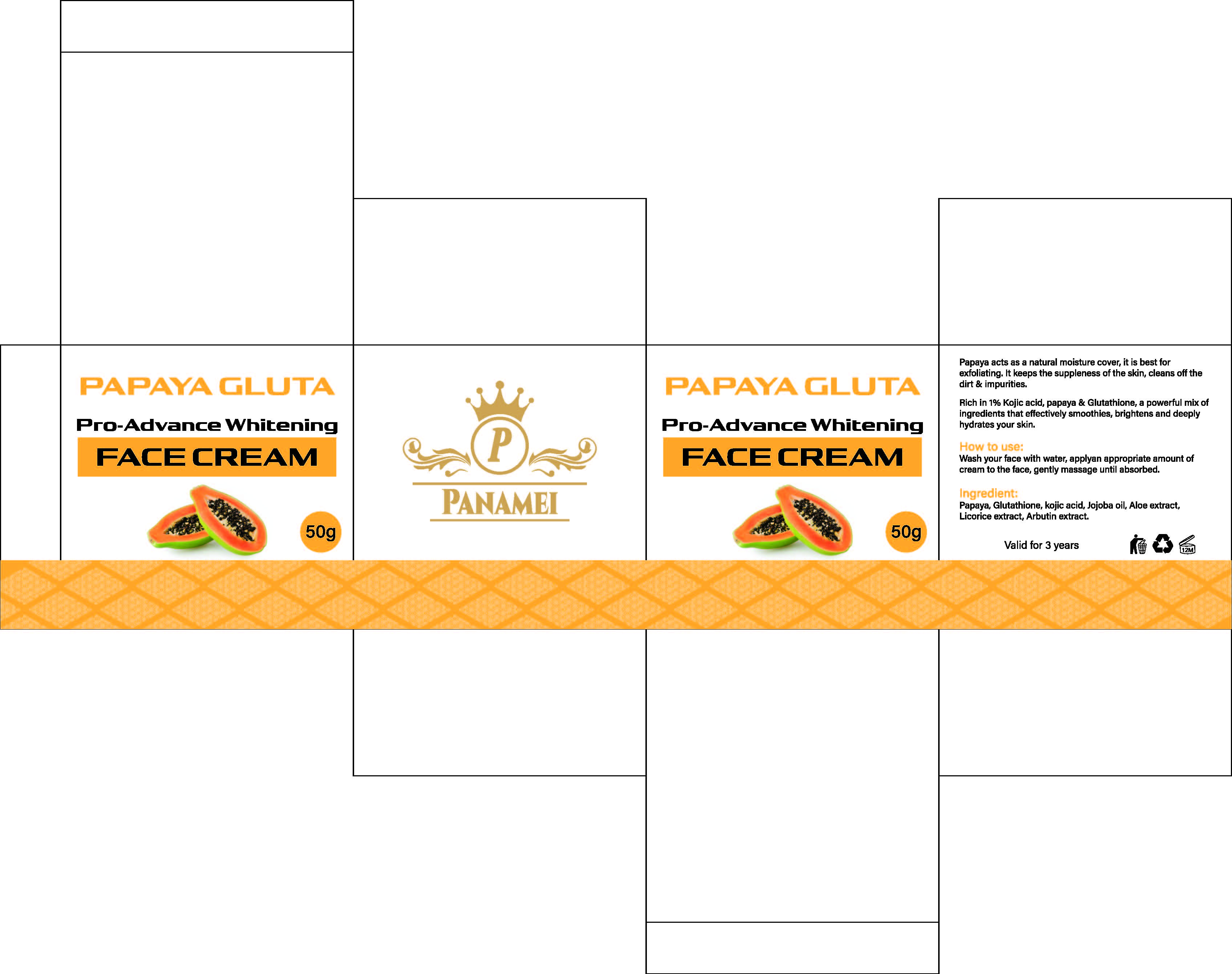
INDICATIONS & USAGE SECTION
Wash vour face with water, applvan appropriate amount ofcream to the face, gently massage until absorbed.
OTC - PURPOSE SECTION
For Facial Skin Moisturizing, Antioxidant, Blemish Removal, Whitening, Brightening
INACTIVE INGREDIENT SECTION
AQUA,MINERAL OIL,GLYCERIN,Polyethylene,PROPYLENE GLYCOL,STEARYL ALCOHOL,BUTYLENE GLYCOL,Steareth-2,Steareth-21,CETYL ALCOHOL,DIMETHICONE,Isosorbide Dimethyl Ether,GLYCERYL STEARATE,PEG-100,PHENOXYETHANOL,ALLANTOIN,Polyacrylamide,ALOE BARBADENSIS LEAF EXTRACT,ANTHEMIS NOBILIS FLOWER EXTRACT,PORTULACA OLERACEA FLOWER/LEAF/STEM EXTRACT,PELARGONIUM GRAVEOLENS FLOWER/LEAF/STEM EXTRACT,XANTHAN GUM,FRAGRANCE,ETHYLHEXYLGLYCERIN, C13-14 Isoparaffin,DISODIUM EDTA,Laureth-7
WARNINGS SECTION
1.Before use, perform a skin test on your arm and discontinue use if you have allergy symptoms or skin abnormalities.
2、For external use only, avoid contact with eyes when used
3、Keep out of reach of children
DOSAGE & ADMINISTRATION SECTION
Gently apply a little bit with your hands, and then spread it evenly.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
KEEP OUT OF REACH OF CHILDREN SECTION
OTC - ACTIVE INGREDIENT SECTION
CARICA PAPAYA FRUIT EXTRACT
