ISOVUE
ISOVUE-200Iopamidol Injection 41%ISOVUE-250Iopamidol Injection 51%ISOVUE-300Iopamidol Injection 61%ISOVUE-370Iopamidol Injection 76%
ae8c18c9-3e7d-4515-b980-120025a88fc1
HUMAN PRESCRIPTION DRUG LABEL
Apr 1, 2023
BRACCO DIAGNOSTICS INC
DUNS: 849234661
Products 4
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
IOPAMIDOL
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (3)
IOPAMIDOL
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (3)
IOPAMIDOL
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (3)
IOPAMIDOL
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (3)
Drug Labeling Information
DESCRIPTION SECTION
DESCRIPTION
ISOVUE (lopamidol Injection) formulations are stable, aqueous, sterile, and nonpyrogenic solutions for intravascular administration.
Each mL of ISOVUE-200 (lopamidol Injection 41%) provides 408 mg iopamidol with 1 mg tromethamine and 0.26 mg edetate calcium disodium. The solution contains approximately 0.029 mg (0.001 mEq) sodium and 200 mg organically bound iodine per mL.
Each mL of ISOVUE-250 (lopamidol Injection 51%) provides 510 mg iopamidol with 1 mg tromethamine and 0. 33 mg edetate calcium disodium. The solution contains approximately 0.036 mg (0.002 mEq) sodium and 250 mg organically bound iodine per mL.
Each mL of ISOVUE-300 (lopamidol Injection 61%) provides 612 mg iopamidol with 1 mg tromethamine and 0.39 mg edetate calcium disodium. The solution contains approximately 0.043 mg (0.002 mEq) sodium and 300 mg organically bound iodine per mL.
Each mL of ISOVUE-370 (lopamidol Injection 76%) provides 755 mg iopamidol with 1 mg tromethamine and 0.48 mg edetate calcium disodium. The solution contains approximately 0.053 mg (0.002 mEq) sodium and 370 mg organically bound iodine per mL.
The pH of ISOVUE contrast media has been adjusted to 6.5-7.5 with hydrochloric acid and/or sodium hydroxide. Pertinent physicochemical data are noted below. ISOVUE (lopamidol Injection) is hypertonic as compared to plasma and cerebrospinal fluid (approximately 285 and 301 mOsm/kg water, respectively).
|
Iopamidol | ||||
|---|---|---|---|---|
|
Parameter |
41% |
51% |
61% |
76% |
|
Concentration |
200 |
250 |
300 |
370 |
|
Osmolality @ 37° C |
413 |
524 |
616 |
796 |
|
Viscosity (cP) @ 37° C |
2.0 |
3.0 |
4.7 |
9.4 |
|
@ 20° C |
3.3 |
5.1 |
8.8 |
20.9 |
|
Specific Gravity @ 37° C |
1.227 |
1.281 |
1.339 |
1.405 |
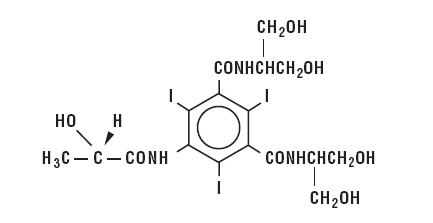
lopamidol is designated chemically as (S)-N,N’-bis[2-hydroxy-1-(hydroxymethyl)-ethyl]-2,4,6-triiodo-5-lactamidoisophthalamide. Structural formula:
|
|
MW 777.09 |
HOW SUPPLIED SECTION
HOW SUPPLIED
ISOVUE-200 (lopamidol Injection 41%)
Ten 200 mL single dose bottles (NDC 0270-1314-15)
ISOVUE-250 (lopamidol Injection 51%)
Ten 100 mL single dose bottles (NDC 0270-1317-02)
ISOVUE-300 (lopamidol Injection 61%)
Ten 30 mL single dose vials (NDC 0270-1315-25)
Ten 50 mL single dose vials (NDC 0270-1315-30)
Ten 100 mL single dose bottles (NDC 0270-1315-35)
Ten 150 mL single dose bottles (NDC 0270-1315-50)
ISOVUE-370 (lopamidol Injection 76%)
Ten 50 mL single dose vials (NDC 0270-1316-30)
Ten 75 mL single dose bottles (NDC 0270-1316-52)
Ten 100 mL single dose bottles (NDC 0270-1316-35)
Ten 125 mL single dose bottles (NDC 0270-1316-04)
Ten 150 mL single dose bottles (NDC 0270-1316-37)
Storage
Store at 20-25° C (68-77° F). [See USP]. Protect from light.
Also Available
lopamidol Injection is also available as ISOVUE-M® for intrathecal administration.
ISOVUE is a registered trademark of Bracco Diagnostics Inc.