ReliBiotic
ReliBiotic
c35d602f-9aa5-4507-8619-4da538fa4789
DIETARY SUPPLEMENT
May 23, 2025
Redmont Pharmaceuticals, LLC
DUNS: 080843607
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
lactobacillus acidophilus and bifidobacterium animalis lactis
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Drug Labeling Information
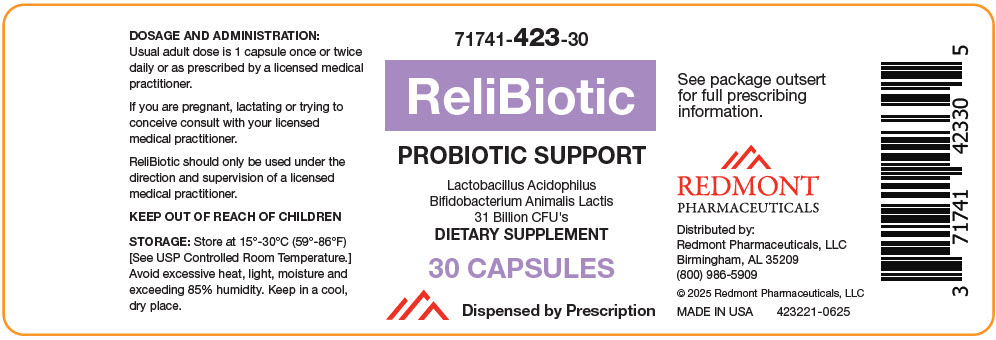
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 30 Capsule Bottle Label
71741-423-30
ReliBiotic
PROBIOTIC SUPPORT
Lactobacillus Acidophilus
Bifidobacterium Animalis Lactis
31 Billion CFU's
DIETARY SUPPLEMENT
30 CAPSULES
Dispensed by Prescription
STATEMENT OF IDENTITY SECTION
|
Supplement Facts | ||
|---|---|---|
|
Serving size 1 Capsule | ||
|
Amount per Serving: |
% Daily Value | |
| ||
|
Lactobacillus Acidophilus |
16 Billion |
|
|
Bifidobacterium Animalis Lactis |
15 Billion |
|
OTHER INGREDIENTS: Modified Cellulose, Silicon Dioxide, Magnesium Stearate.
Preservative Free and Gluten Free
HEALTH CLAIM SECTION
© 2025 Redmont Pharmaceuticals, LLC
423111-0625
WARNINGS SECTION
WARNING
ReliBiotic should only be used under the direction and supervision of a licensed medical practitioner. Use with caution in patients that may have a medical condition, are pregnant, lactating, trying to conceive, under the age of 18, or taking other medications.
DOSAGE & ADMINISTRATION SECTION
DOSAGE AND ADMINISTRATION
Usual adult dose is 1 capsule once or twice daily or as prescribed by a licensed medical practitioner.
SAFE HANDLING WARNING SECTION
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN
