Phendimetrazine Tartrate
PHENDIMETRAZINE TARTRATE TABLETS, USP 35 mg, CIII Rx Only
767b5386-1e56-4eeb-8d59-510b940ab75e
HUMAN PRESCRIPTION DRUG LABEL
Sep 8, 2025
Preferred Pharmaceuticals Inc.
DUNS: 791119022
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Phendimetrazine Tartrate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
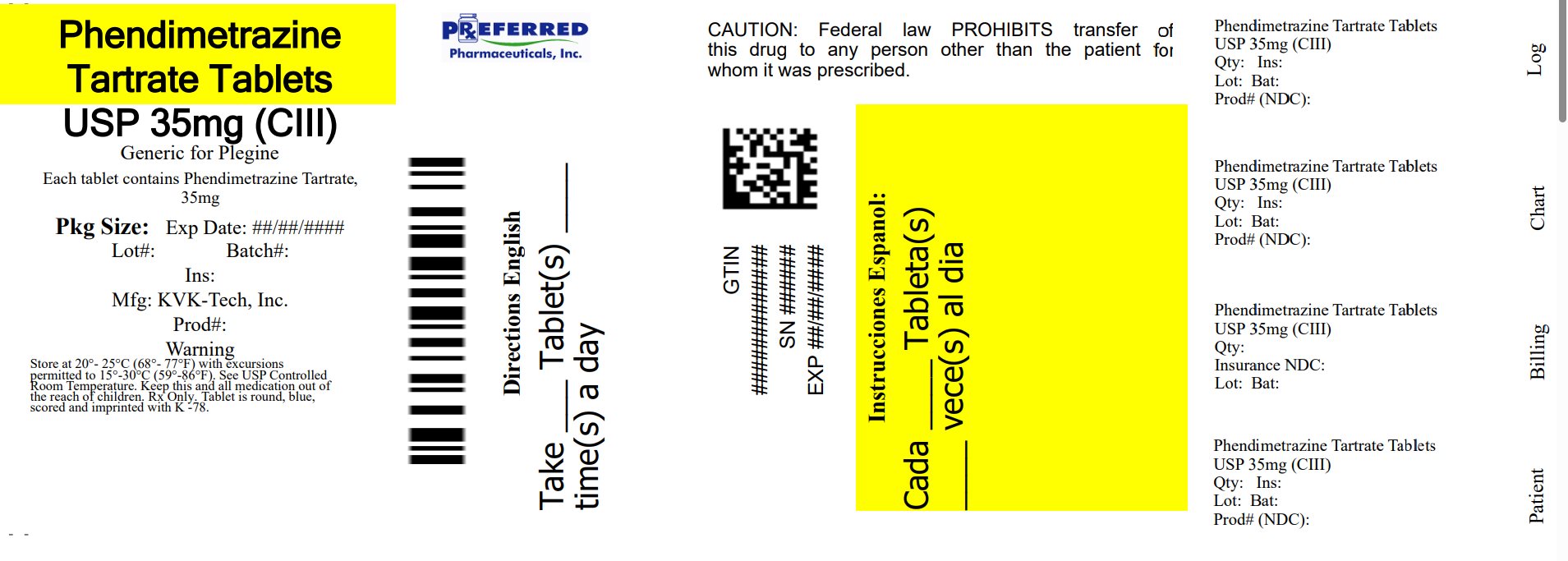
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal Display Panel
NDC 68788-7236
Phendimetrazine Tartrate Tablets, USP
35 mg CIII
Rx Only
KVK-TECH
Repackaged By: Preferred Pharmaceuticals Inc.
INDICATIONS & USAGE SECTION
INDICATIONS AND USAGE:
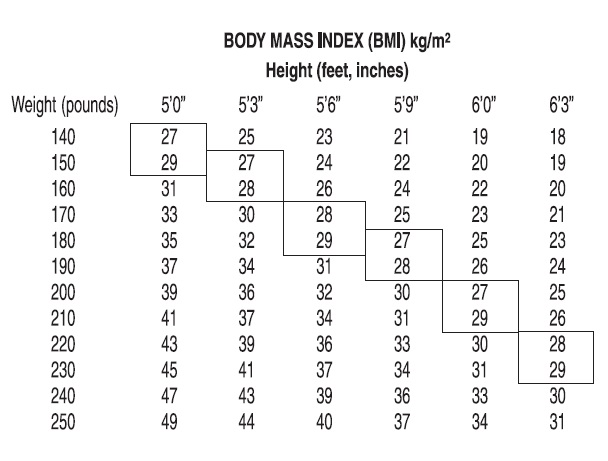
Phendimetrazine tartrate is indicated in the management of exogenous obesity as a short term adjunct (a few weeks) in a regimen of weight reduction based on caloric restriction in patients with an initial body mass index (BMI) of 30 kg/m 2 or higher who have not responded to appropriate weight reducing regimen (diet and/or exercise) alone. Below is a chart of Body Mass Index (BMI) based on various heights and weights. BMI is calculated by taking the patient’s weight, in kilograms (kg), divided by the patient’s height, in meters (m), squared. Metric conversions are as follows: pounds ÷ 2.2 = kg; inches x 0.0254 = meters.
Phendimetrazine tartrate is indicated for use as monotherapy only.
CONTRAINDICATIONS SECTION
CONTRAINDICATIONS:
Known hypersensitivity or idiosyncratic reactions to sympathomimetics.
Advanced arteriosclerosis, symptomatic cardiovascular disease, moderate and severe hypertension, hyperthyroidism, and glaucoma.
Highly nervous or agitated patients.
Patients with a history of drug abuse.
Patients taking other CNS stimulants, including monoamine oxidase inhibitors.
ADVERSE REACTIONS SECTION
ADVERSE REACTIONS:
Cardiovascular: Palpitations, tachycardia, elevated blood pressure, ischemic events.
Valvular heart disease associated with the use of some anorectic agents such as fenfluramine and dexfenfluramine, both independently and especially when used in combination with other anorectic drugs, have been reported. However, no case of this valvulopathy has been reported when phendimetrazine tartrate has been used alone.
Central Nervous System:
Overstimulation, restlessness, insomnia, agitation, flushing, tremor, sweating, dizziness, headache, psychotic state, blurring of vision.
Gastrointestinal:
Dryness of the mouth, nausea, diarrhea, constipation, stomach pain.
Genitourinary:
Urinary frequency, dysuria, changes in libido.
DESCRIPTION SECTION
DESCRIPTION:
Phendimetrazine tartrate, as the dextro isomer, has the chemical name of (2S,3S)-3,4-Dimethyl-2-phenylmorpholine L-(+)- tartrate (1:1).
The structural formula is:

Phendimetrazine tartrate is a white, odorless crystalline powder. It is freely soluble in water; sparingly soluble in warm alcohol, insoluble in chloroform, acetone, ether and benzene.
Each white tablet, for oral administration, contains 35 mg of phendimetrazine tartrate. In addition, the following inactive ingredients are present: Colloidal Silicon Dioxide, Lactose Monohydrate, Microcrystalline Cellulose 102, Sodium Starch Glycolate and Stearic Acid. The yellow tablet also contains FD&C Yellow # 5 Aluminum Lake (15-17%). The blue tablet also contains FD&C Blue # 1 Aluminum Lake (11-13%).
PRECAUTIONS SECTION
PRECAUTIONS:
Caution is to be exercised in prescribing phendimetrazine for patients with even mild hypertension.
Insulin requirements in diabetes mellitus may be altered in association with the use of phendimetrazine tartrate and the concomitant dietary regimen.
Phendimetrazine tartrate may decrease the hypotensive effect of guanethidine. The least amount feasible should be prescribed or dispensed at one time in order to minimize the possibility of overdosage.
Only yellow tablet contains FD&C Yellow No. 5 (tartrazine) which may cause allergic-type reactions (including bronchial asthma) in certain susceptible persons. Although the overall incidence of FD&C Yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity.
Drug Interactions:
Efficacy of phendimetrazine tartrate with other anorectic agents has not been studied and the combined use may have the potential for serious cardiac problems.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Studies with Phendimetrazine Tartrate have not been performed to evaluate carcinogenic potential, mutagenic potential or effects on fertility.
Pregnancy: Pregnancy Category C:
Animal reproduction studies have not been conducted with phendimetrazine tartrate. It is also not known whether phendimetrazine tartrate can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity.
Usage in Pregnancy:
Safe use in pregnancy has not been established. Until more information is available, phendimetrazine tartrate should not be taken by women who are or may become pregnant unless, in the opinion of the physician, the potential benefits outweigh the possible hazards.
Nursing Mothers:
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, phendimetrazine tartrate should not be taken by women who are nursing unless, in the opinion of the physician, the potential benefits outweigh the possible hazards.
Pediatric Use:
Safety and effectiveness in pediatric patients have not been established.
OVERDOSAGE SECTION
OVERDOSAGE:
Acute overdosage with phendimetrazine tartrate may manifest itself by the following signs and symptoms: unusual restlessness, confusion, belligerence, hallucinations, and panic states. Fatigue and depression usually follow the central stimulation. Cardiovascular effects include arrhythmias, hypertension, or hypotension and circulatory collapse. Gastrointestinal symptoms include nausea, vomiting, diarrhea, and abdominal cramps. Poisoning may result in convulsions, coma, and death.
The management of overdosage is largely symptomatic. It includes sedation with a barbiturate. If hypertension is marked, the use of a nitrate or rapid-acting alpha receptor-blocking agent should be considered. Experience with hemodialysis or peritoneal dialysis is inadequate to permit recommendations for its use.
CLINICAL PHARMACOLOGY SECTION
CLINICAL PHARMACOLOGY:
Phendimetrazine tartrate is a sympathomimetic amine with pharmacological activity similar to the prototype drugs of this class used in obesity, the amphetamines. Actions include central nervous system stimulation and elevation of blood pressure. Tachyphylaxis and tolerance have been demonstrated with all drugs of this class in which these phenomena have been looked for.
Drugs of this class used in obesity are commonly known as “anorectics” or “anorexigenics”. It has not been established, however, that the action of such drugs in treating obesity is primarily one of appetite suppression. Other central nervous system actions or metabolic effects, may be involved for example.
Adult obese subjects instructed in dietary management and treated with anorectic drugs, lose more weight on the average than those treated with placebo and diet, as determined in relatively short term clinical trials.
The magnitude of increased weight loss of drug-treated patients over placebo- treated patients is only a fraction of a pound a week. The rate of weight loss is greatest in the first weeks of therapy for both drug and placebo subjects and tends to decrease in succeeding weeks. The possible origin of the increased weight loss due to the various drug effects is not established. The amount of weight loss associated with the use of an anorectic drug varies from trial to trial, and the increased weight loss appears to be related in part to variables other than the drug prescribed, such as the physician investigator, the population treated, and the diet prescribed. Studies do not permit conclusions as to the relative importance of the drug and non-drug factors on weight loss.
The natural history of obesity is measured in years, whereas the studies cited are restricted to a few weeks duration; thus, the total impact of drug-induced weight loss over that of diet alone must be considered clinically limited.
The major route of elimination is via the kidneys where most of the drug and metabolites are excreted. Some of the drug is metabolized to phendimetrazine and phendimetrazine-N-oxide. The average half-life of elimination when studied under controlled conditions is about 3.7 hours for both the extended-release and immediate release forms. The absorption half-life of the drug from the immediate release 35 mg phendimetrazine tablets is appreciably more rapid than the absorption rate of the drug from the extended-release formulation.
DRUG ABUSE AND DEPENDENCE SECTION
DRUG ABUSE AND DEPENDENCE:
Controlled Substance:
Phendimetrazine tartrate tablets are a Schedule lll controlled substance.
Dependence:
Phendimetrazine tartrate is related chemically and pharmacologically to the amphetamines. Amphetamines and related stimulant drugs have been extensively abused, and the possibility of abuse of phendimetrazine should be kept in mind when evaluating the desirability of including a drug as part of a weight reduction program. Abuse of amphetamines and related drugs may be associated with intense psychological dependence and severe social dysfunction. There are reports of patients who have increased the dosage to many times that recommended. Abrupt cessation following prolonged high dosage administration results in extreme fatigue and mental depression; changes are also noted on the sleep EEG. Manifestations of chronic intoxication with anorectic drugs include severe dermatoses, marked insomnia, irritability, hyperactivity and personality changes. The most severe manifestation of chronic intoxications is psychosis, often clinically indistinguishable from schizophrenia.
WARNINGS SECTION
WARNINGS:
Phendimetrazine tartrate should not be used in combination with other anorectic agents, including prescribed drugs, over-the-counter preparations and herbal products.
In a case-control epidemiological study, the use of anorectic agents, including phendimetrazine tartrate, was associated with an increased risk of developing pulmonary hypertension, a rare, but often fatal disorder. The use of anorectic agents for longer than three months was associated with a 23-fold increase in the risk of developing pulmonary hypertension. Increased risk of pulmonary hypertension with repeated courses of therapy cannot be excluded.
The onset or aggravation of exertional dyspnea, or unexplained symptoms of angina pectoris, syncope, or lower extremity edema suggest the possibility of occurrence of pulmonary hypertension. Under these circumstances, phendimetrazine tartrate should be immediately discontinued, and the patient should be evaluated for the possible presence of pulmonary hypertension.
Valvular heart disease associated with the use of some anorectic agents such as fenfluramine and dexfenfluramine has been reported. Possible contributing factors include use for extended periods of time, higher than recommended dose, and/or use in combination with other anorectic drugs. However, no cases of this valvulopathy have been reported when phendimetrazine tartrate has been used alone.
The potential risk of possible serious adverse effects such as valvular heart disease and pulmonary hypertension should be assessed carefully against the potential benefit of weight loss. Baseline cardiac evaluation should be considered to detect pre-existing valvular heart diseases or pulmonary hypertension prior to initiation of phendimetrazine treatment. Phendimetrazine tartrate is not recommended in patients with known heart murmur or valvular heart disease. Echocardiogram during and after treatment could be useful for detecting any valvular disorders which may occur. To limit unwarranted exposure and risks, treatment with phendimetrazine tartrate should be continued only if the patient has satisfactory weight loss within the first 4 weeks of treatment (i.e., weight loss of at least 4 pounds, or as determined by the physician and patient).
Tolerance to the anorectic effect of phendimetrazine develops within a few weeks. When this occurs, its use should be discontinued; the maximum recommended dose should not be exceeded.
Use of phendimetrazine tartrate within 14 days following the administration of monoamine oxidase inhibitors may result in a hypertensive crisis.
Abrupt cessation of administration following prolonged high dosage results in extreme fatigue and depression. Because of the effect on the central nervous system, phendimetrazine tartrate may impair the ability of the patient to engage in potentially hazardous activities such as operating machinery or driving a motor vehicle; the patient should therefore be cautioned accordingly.
Phendimetrazine tartrate is not recommended for patients who used any anorectic agents within the prior year.
DOSAGE & ADMINISTRATION SECTION
DOSAGE AND ADMINISTRATION:
Usual Adult Dosage: 1 tablet (35 mg) twice a day or three times a day one hour before meals.
Dosage should be individualized to obtain an adequate response with the lowest effective dosage. In some cases, ½ tablet (17.5 mg) per dose may be adequate. Dosage should not exceed 2 tablets three times a day.
HOW SUPPLIED SECTION
HOW SUPPLIED:
Phendimetrazine Tartrate Tablets, USP 35 mgare available as
Blue, round, biconvex tablets debossed “K” above bisect “78” on one side and plain on the other side.
Bottles of 7 NDC 68788-7236-7
Bottles of 17 NDC 68788-7236-1
Bottles of 28 NDC 68788-7236-2
Bottles of 30 NDC 68788-7236-3
SPL UNCLASSIFIED SECTION
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature].
DEA Order Form Required.
Manufactured by:
KVK-TECH, INC.
110 Terry Drive Suite 200
Newtown, PA 18940-1850
Item ID # 006080/09 09/2019
Manufacturer’s Code: 10702
Repackaged By: Preferred Pharmaceuticals Inc.
