QUININE SULFATE
These highlights do not include all the information needed to use Quinine Sulfate Capsules USP safely and effectively. See full prescribing information for quinine sulfate capsules USP.QUININE Sulfate Capsules, USP for Oral useInitial U.S. Approval: 2005
1f32f05c-a215-40be-b951-e41a7b54a8a0
HUMAN PRESCRIPTION DRUG LABEL
Sep 16, 2025
Lupin Pharmaceuticals, Inc.
DUNS: 089153071
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
QUININE SULFATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Drug Labeling Information
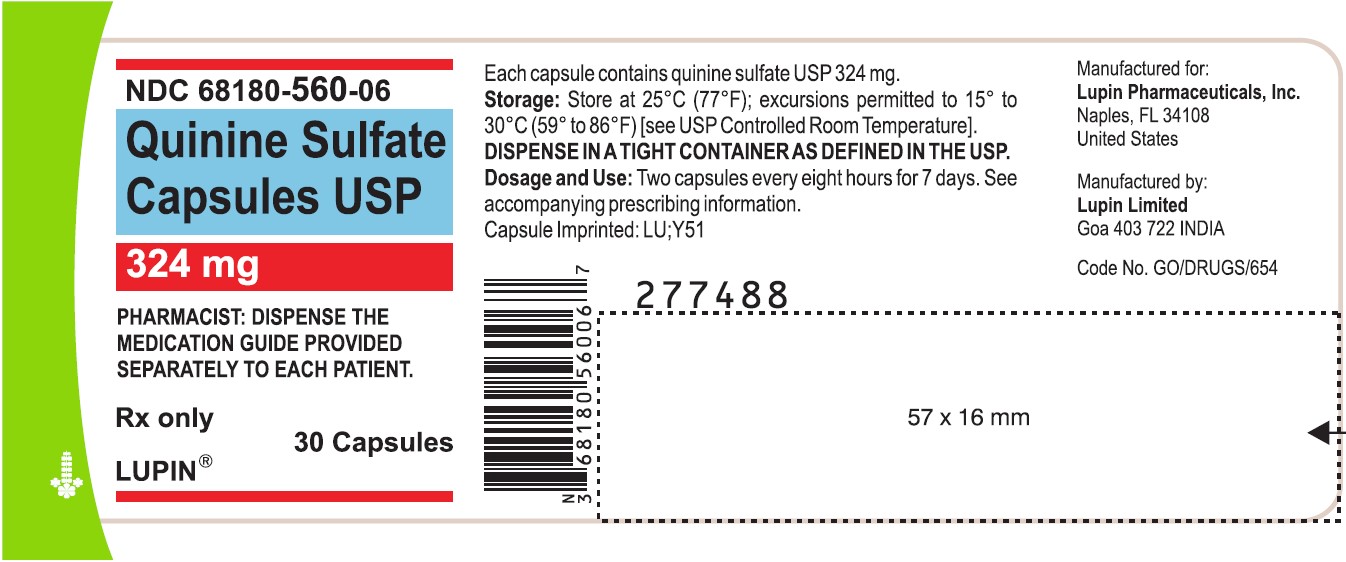
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
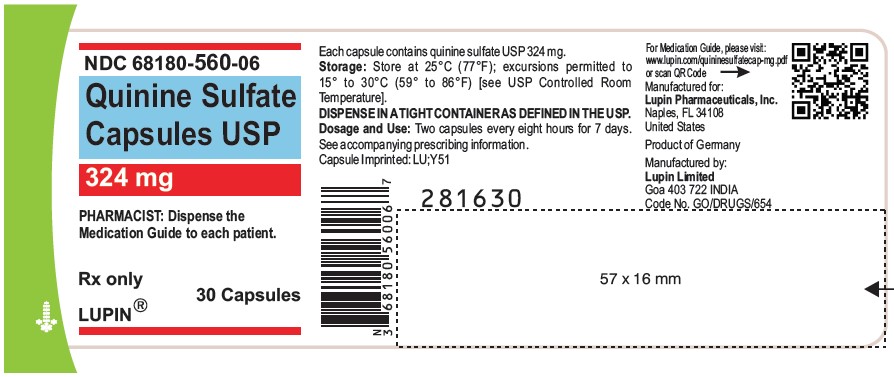
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Quinine Sulfate Capsules USP
Rx Only
324 mg
NDC-68180-560-06
30 Capsules
DESCRIPTION SECTION
11 DESCRIPTION
Quinine sulfate is a cinchona alkaloid chemically described as cinchonan-9-ol, 6'-methoxy-, (8α, 9R)-, sulfate (2:1) (salt), dihydrate with a molecular formula of (C20H24N2O2)2•H2SO4•2H2O and a molecular weight of 782.94.
The structural formula of quinine sulfate is:
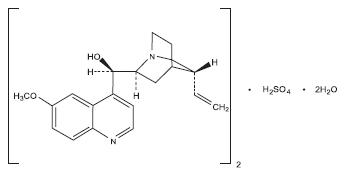
Quinine sulfate occurs as a white, crystalline powder that darkens on exposure to light. It is odorless and has a persistent very bitter taste. It is only slightly soluble in water, alcohol, chloroform, and ether.
Quinine sulfate capsules USP are supplied for oral administration as capsules containing 324 mg of the active ingredient quinine sulfate USP, equivalent to 269 mg free base. Inactive ingredients: black iron oxide, corn starch, gelatin, magnesium stearate, potassium hydroxide, propylene glycol shellac, sodium lauryl sulfate, and talc.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Quinine sulfate capsules USP, 324 mg are available as size "0" capsules with clear transparent cap and clear transparent body imprinted with "LU" on cap and "Y51" on body in black ink, containing white to off white powder:
Bottles of 30 NDC 68180-560-06
16.2 Storage
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F).
[See USP Controlled Room Temperature]
Dispense in a tight container as defined in the USP.
SPL MEDGUIDE SECTION
MEDICATION GUIDE
Quinine Sulfate Capsules USP
(Kwye' nine sul' fate)
Read the Medication Guide that comes with quinine sulfate capsules before you start taking it and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking to your healthcare provider about your medical condition or treatment. You and your healthcare provider should talk about quinine sulfate capsules when you start taking it and at regular checkups. Quinine sulfate capsules are not approved for the treatment of night-time leg cramps.
What is the most important information I should know about quinine sulfate capsules?
Quinine sulfate capsules used to treat or prevent leg cramps may cause serious side effects or even death.
- Quinine sulfate capsules may cause your blood cell (platelet) count to drop causing serious bleeding problems. In some people, serious kidney problems can happen.
- Quinine sulfate capsules may cause problems with your heart rhythm that can lead to death.
- Quinine sulfate capsules may cause serious allergic reactions.
Call your healthcare provider right away if you have:
- easy bruising
- severe nose bleed
- blood in urine or stool
- bleeding gums
- appearance of unusual purple, brown or red spots on your skin (bleeding under your skin)
- rash
- hives
- severe itching
- severe flushing
- swelling of your face
- trouble breathing
- chest pain
- rapid heartbeat
- irregular heart rhythm
- weakness
- sweating
- nervousness
Taking quinine sulfate capsules with some other medicines can increase the chance of serious side effects. Tell your health care provider if you take any other medicines.
Certain medicines can cause the blood levels of quinine sulfate to be too high or too low in your body.It is important for you to tell your healthcare provider about all the medicines you take, including prescription and non- prescription medicines, vitamins and herbal supplements.
Quinine sulfate capsules and other medicines may affect each other causing serious side effects or death. Even medicines that you may take for a short period of time, such as antibiotics, can mix in your blood with quinine sulfate and cause serious side effects or death.Do not start taking a new medicine without telling your healthcare provider or pharmacist.
What are quinine sulfate capsules?
Quinine sulfate capsule is a prescription medication used to treat malaria (uncomplicated) caused by the parasite Plasmodium falciparum.
Quinine sulfate capsules are Not approved to:
- Prevent malaria
- Treat severe or complicated malaria
- Prevent or treat night-time leg cramps
It is not known if quinine sulfate capsules are safe and works in children younger than 16 years old.
Who should not take quinine sulfate capsules?
Do not take quinine sulfate capsules if you have:
- certain heart rhythm problems (atrial fibrillation) or abnormal electrocardiogram (ECG) (QT prolongation).
- low levels of an enzyme called Glucose-6-phosphate dehydrogenase (G6PD).
- an autoimmune disease (myasthenia gravis) that leads to muscle weakness.
- had allergic reactions to quinine, quinidine, or mefloquine (Lariam®).
- had serious side effects to quinine, such as low platelets, which are necessary for your blood to clot.
- an inflammation of the nerve important for vision (optic neuritis).
What should I tell my healthcare provider before starting quinine sulfate capsules?
Before you take quinine sulfate capsules, tell your health-care provider if you:
- Have heart problems.
- Have kidney problems.
- Have liver problems.
- Have any other medical condition.
- Are pregnant or could be pregnant. Treatment of malaria is important because it can be a serious disease for a pregnant woman and her unborn baby. Your healthcare provider can tell you more about the benefits and risks of taking this medication during pregnancy. Low blood sugar (hypoglycemia) can be seen in pregnant women while taking quinine sulfate capsules. This can include sweating, weakness, nausea, vomiting, or confusion. You and your healthcare provider can decide if quinine sulfate capsules are right for you.
- Are breast-feeding. Small amounts of quinine sulfate can pass into your breast milk. You and your healthcare provider can decide if you should breastfeed while taking quinine sulfate capsules.
Tell your healthcare provider about all the medicines you take, including prescription medicines, vitamins and herbal supplements.
How should I take quinine sulfate capsules?
- Take quinine sulfate capsules USP exactly as your healthcare provider tells you to take it.
- Your healthcare provider will tell you how many quinine sulfate capsules to take and when to take them.
- To lower the chance of stomach upset, take quinine sulfate capsuleswith food .
- Finish all the quinine sulfate capsules that is prescribed even if you feel better.Do not stop taking the medication without talking to your healthcare provider. *Do not take more than the amount prescribed.Do not take more than 2 capsules at one time or more than 3 doses in one day. If you take more than the prescribed dose, call your healthcare provider right away.
- If you forget to take quinine sulfate capsules,do not double the next dose. If it has been more than 4 hours since the missed dose, just wait and take the regular dose at the next scheduled time. Call your healthcare provider if you are not sure what to do.
- If you take too much quinine sulfate capsules, call your healthcare provider or go to the nearest emergency room right away.
Call your healthcare provider right away if:
- If you feel worse, or if you do not start feeling better within 1 or 2 days of starting to take quinine sulfate capsules.
- If your fever comes back after finishing treatment with quinine sulfate capsules.
What are the possible side effects of quinine sulfate capsules?
Quinine sulfate capsules may cause serious side effects.
- See "What is the most important information I should know about quinine sulfate capsules**"** section.
- Low blood sugar (hypoglycemia). This can include sweating, weakness, nausea, vomiting, or confusion.
Common side effects with quinine sulfate capsules include:
- headache
- sweating
- flushing
- nausea
- ringing in your ears
- diarrhea
- deafness
- hearing loss
- dizziness (vertigo)
- blurred vision
- changes in how you see color
- vomiting
- stomach pain
- blindness
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all of the possible side effects of quinine sulfate capsules. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store quinine sulfate capsules?
- Keep the capsules in a tightly closed container.
- Do not refrigerate or freeze.
- Store at 15° to 30°C (59° to 86°F).
Keep quinine sulfate capsules and all medicines out of the reach of children.
General Information about quinine sulfate capsules
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use quinine sulfate capsules for a condition for which it was not prescribed. Do not give quinine sulfate capsules to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about quinine sulfate capsules. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about quinine sulfate capsules that is written for healthcare professionals.
For more information, go to www.lupinpharmaceuticals.com or call 1-800-399-2561.
What are the ingredients in quinine sulfate capsules?
**Active Ingredients:**Quinine Sulfate USP
**Inactive Ingredients:**Black Iron Oxide, Corn Starch, Gelatin, Magnesium Stearate, Potassium Hydroxide, Propylene Glycol, Shellac, Sodium Lauryl Sulfate and Talc.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
LUPIN and
the are
registered trademarks of Lupin Pharmaceuticals, Inc.
are
registered trademarks of Lupin Pharmaceuticals, Inc.
Manufactured for:
Lupin Pharmaceuticals, Inc.
Naples, FL 34108
United States.
Manufactured by:
Lupin Limited
Goa 403 722
INDIA.
September 2024 ID#: 277505
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
SeeFDA-approved****patient labelling (Medication Guide)
17.1 Dosing Instructions
Patients should be instructed to:
- Take all of the medication as directed.
- Take no more of the medication than the amount prescribed.
- Take with food to minimize possible gastrointestinal irritation.
If a dose is missed, patients should also be instructed not to double the next dose. If more than 4 hours has elapsed since the missed dose, the patient should wait and take the next dose as previously scheduled.
LUPIN and
the are
registered trademarks of Lupin Pharmaceuticals, Inc.
are
registered trademarks of Lupin Pharmaceuticals, Inc.
Manufactured for:
Lupin Pharmaceuticals, Inc.
Naples, FL 34108
United States.
Manufactured by:
Lupin Limited
Goa 403 722
INDIA.
September 2024 ID#: 277504
