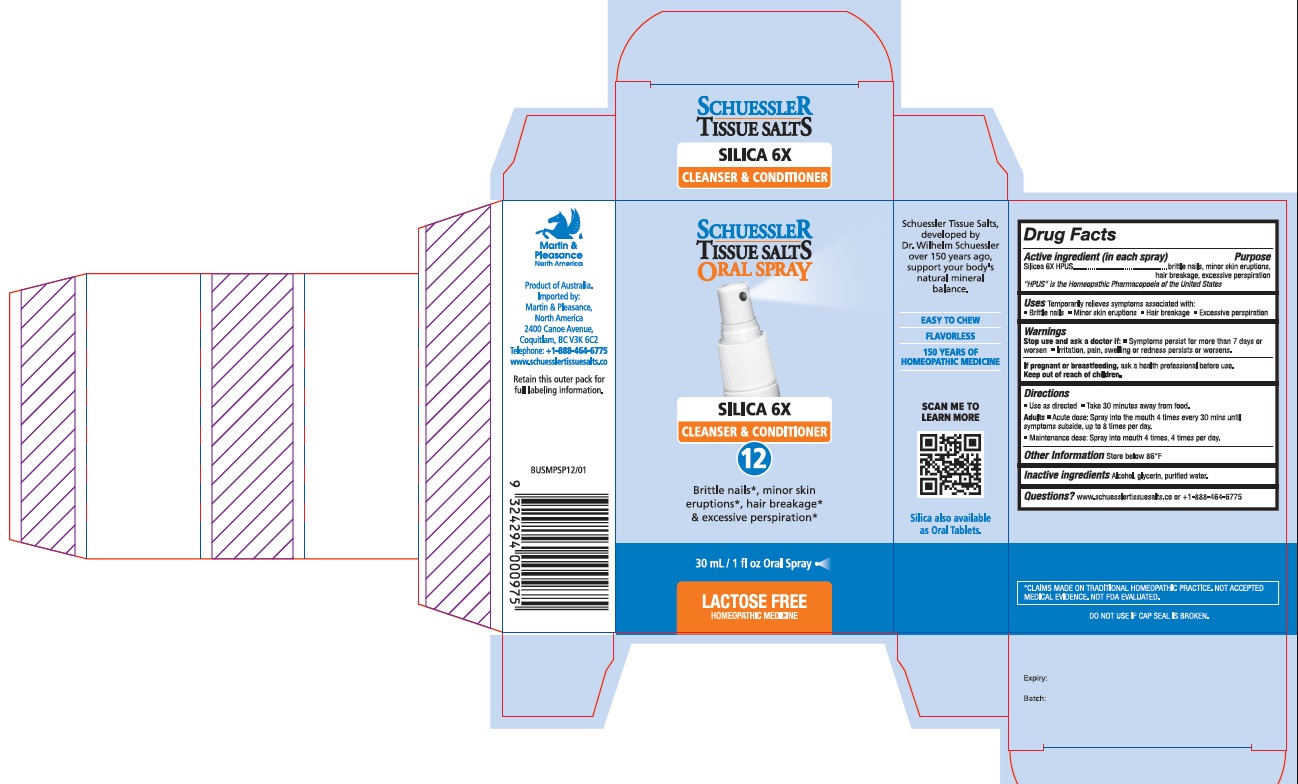
Schuessler Tissue Salts Silica 6X Cleanser and Conditioner 12
39620db9-5099-47f0-93ad-c00e6625c462
HUMAN OTC DRUG LABEL
Jul 7, 2025
Martin & Pleasance Pty Ltd
DUNS: 752869859
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Silicea
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
Drug Labeling Information
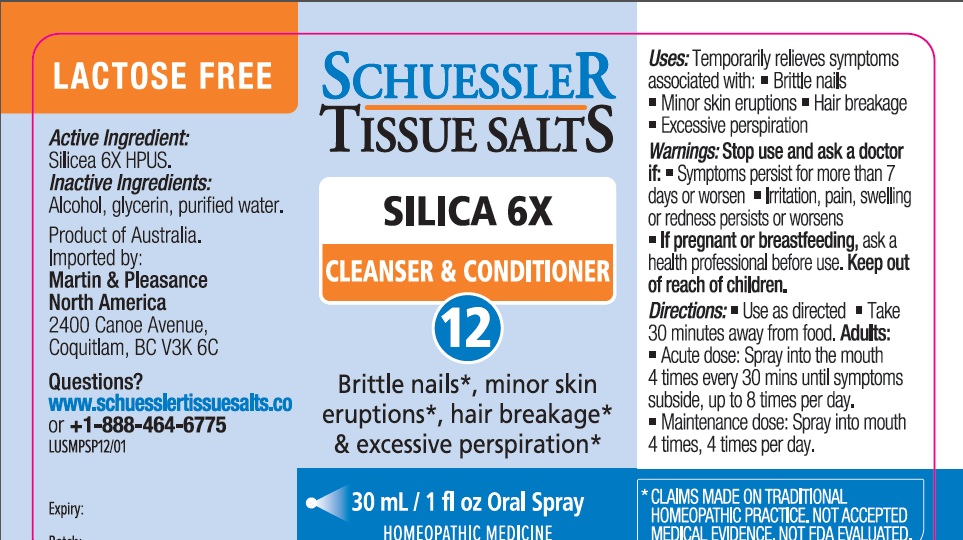
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Product label
INDICATIONS & USAGE SECTION
Uses
Temporarily relieves symptoms associated with:
• Brittle nails
• Minor skin eruptions
• Hair breakage
• Excessive perspiration
OTC - ACTIVE INGREDIENT SECTION
Active ingredient (in each spray) Purpose
Silicea 6X HPUS brittle nails, minor skin eruptions,hair breakage, excessive perspiration
"HPUS" is the Homeopathic Pharmacopoeia of the United States
OTC - PURPOSE SECTION
WARNINGS SECTION
Warnings
Stop use and ask a doctor if:
- Symptoms persist for more than 7 days or worsen
- Irritation, pain, swelling or redness persists or worsens.
If pregnant or breastfeeding, ask a health professional before use.Keep out of reach of children.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
DOSAGE & ADMINISTRATION SECTION
Directions
- Use as directed
- Take 30 minutes away from food.
Adults
- Acute dose: Spray into the mouth 4 times every 30 mins until symptoms subside, up to 8 times per day.
- Maintenance dose: Spray into mouth 4 times, 4 times per day.
SPL UNCLASSIFIED SECTION
**Questions?**www.schuesslertissuesalts.co or + 1-888-464-6775
INACTIVE INGREDIENT SECTION
Inactive Ingredients
Alcohol, Glycerin, Purified Water
