oxaliplatin
These highlights do not include all the information needed to use OXALIPLATIN INJECTION safely and effectively. See full prescribing information for OXALIPLATIN INJECTION. OXALIPLATIN injection, for intravenous use Initial U.S. Approval: 2002
29a5d098-bfa7-4a65-bb4f-5daa1fef1c2f
HUMAN PRESCRIPTION DRUG LABEL
Sep 9, 2025
Sagent Pharmaceuticals
DUNS: 080579617
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
oxaliplatin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – Vial Label
NDC 25021-233-20
Rx only
OXALIPLATIN Injection, USP
100 mg per 20 mL (5 mg per mL)
For Intravenous Use Only
20 mL Single-Dose Vial
See package insert for further required dilution
DO NOT MIX OR ADD TO SODIUM CHLORIDE/
CHLORIDE-CONTAINING SOLUTIONS
Caution: Cytotoxic Agent
SPL PATIENT PACKAGE INSERT SECTION
FDA-Approved Patient Labeling
|
This Patient Information has been approved by the U.S. Food and Drug Administration. | |
|
Patient Information | |
|
What is the most important information I should know about Oxaliplatin Injection? Oxaliplatin Injection can cause serious allergic reactions, including
allergic reactions that can lead to death. Oxaliplatin Injection is a
platinum-based medicine. Serious allergic reactions including death can happen
in people who take Oxaliplatin Injection and who have had previous allergic
reactions to platinum-based medicines. Serious allergic reactions can happen
within a few minutes of your Oxaliplatin Injection infusion or any time during
your treatment with Oxaliplatin Injection. *have trouble breathing *feel like your throat is closing up *chest tightness Call your doctor right away if you have any of the following signs or symptoms of an allergic reaction: | |
|
|
|
See**“What are the possible side effects of Oxaliplatin Injection?”** for information about other serious side effects. | |
|
What is Oxaliplatin Injection?
It is not known if Oxaliplatin Injection is safe and effective in children. | |
|
Do not receive Oxaliplatin Injection if you are allergic to oxaliplatin or any of the ingredients in Oxaliplatin Injection or if you are allergic to other platinum-based medicines. See the end of this leaflet for a complete list of the ingredients in Oxaliplatin Injection. Ask your doctor if you are not sure if you have taken a platinum-based medicine. | |
|
Before receiving Oxaliplatin Injection, tell your doctor about all of your medical conditions, including if you:
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Know the medicines you take. Keep a list of them and show it to your doctor and pharmacist when you get a new medicine. | |
|
How will I receive Oxaliplatin Injection?
Treatment Day 1:
Treatment Day 2: The fluorouracil will be given through your IV with a pump. If you have any problems with the pump or the tube, call your doctor, your nurse, or the person who is responsible for your pump. Do not let anyone other than a healthcare provider touch your infusion pump or tubing. | |
|
What should I avoid while receiving Oxaliplatin Injection?
See**“How can I reduce the side effects caused by cold temperatures?”** for more information. Talk with your doctor and nurse about your level of activity during treatment with Oxaliplatin Injection. Follow their instructions. | |
|
What are the possible side effects of Oxaliplatin Injection? Oxaliplatin Injection can cause serious side effects, including:
For information on ways to lessen or help with nerve problems, see the section “How can I reduce the side effects caused by cold temperatures?” below. *Severe low blood cell counts (myelosuppression). Oxaliplatin Injection when used with fluorouracil and leucovorin can cause low blood cells counts. Low blood cell counts are common with Oxaliplatin Injection when used with fluorouracil and leucovorin and can lead to serious infection and death. Your doctor will do blood tests to check your blood cell counts before starting Oxaliplatin Injection and during treatment. Tell your doctor right away if you have a fever greater than 100.9°F (38.3°C) or a prolonged fever greater than 100.4°F (38°C) for more than one hour (febrile neutropenia). Call your doctor right away if you get any of the following signs of infection: | |
|
|
|
*Posterior Reversible Encephalopathy Syndrome (PRES). PRES is a rare condition that affects the brain. Tell your doctor right away if you have any of the following signs and symptoms of PRES: * headache * confusion or a change in the way you think * seizures * vision problems, such as blurriness or vision loss *Lung problems. Oxaliplatin Injection can cause lung problems that may lead to death. Tell your doctor right away if you get any of the following symptoms as these may be indicators of a serious lung disease: * shortness of breath * wheezing * cough *Liver problems (hepatotoxicity). Your doctor will do blood tests to check your liver when you start receiving Oxaliplatin Injection, and before each treatment course as needed. *Heart problems. Oxaliplatin Injection can cause heart problems that have led to death. Your doctor may do blood and heart tests during treatment with Oxaliplatin Injection if you have certain heart problems. If you faint (lose consciousness), or have an irregular heartbeat or chest pain during treatment with Oxaliplatin Injection, get medical help right away as this may be a sign of a serious heart condition. *Muscle problems. Oxaliplatin Injection can cause muscle damage (rhabdomyolysis) which can lead to death. Tell your doctor right away if you have muscle pain and swelling, along with weakness, fever, red-brown urine, decreased amount of urine or trouble urinating. *Bleeding problems (hemorrhage). Oxaliplatin Injection when used with fluorouracil and leucovorin can cause bleeding problems (hemorrhage) that can lead to death. Your risk of bleeding may increase if you are also taking a blood thinner medicine. Tell your healthcare provider if you have any signs or symptoms of bleeding, including: | |
|
|
|
The most common side effects of Oxaliplatin Injection include: | |
|
|
|
Oxaliplatin Injection may cause fertility problems in males and females. Talk to your doctor if this is a concern for you. Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of Oxaliplatin Injection. For more information, ask your doctor or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | |
|
How can I reduce the side effects caused by cold temperatures?
Your doctor may have other useful tips for helping you with side effects. | |
|
General information about the safe and effective use of Oxaliplatin
Injection. | |
|
What are the ingredients in Oxaliplatin Injection? sagent® Revised: May 2025 |
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
See FDA-Approved Patient Information.
Hypersensitivity Reactions
Advise patients of the potential risk of hypersensitivity and that oxaliplatin injection is contraindicated in patients with a history of hypersensitivity reactions to oxaliplatin and other platinum-based drugs. Instruct patients to seek immediate medical attention for signs of severe hypersensitivity reaction such as chest tightness; shortness of breath; wheezing; dizziness or faintness; or swelling of the face, eyelids, or lips [see Warnings and Precautions (5.1)].
Peripheral Sensory Neuropathy
Advise patients of the risk of acute reversible or persistent-type neurosensory toxicity. Advise patients to avoid cold drinks, use of ice, and exposure of skin to cold temperature or cold objects [see Warnings and Precautions (5.2)].
Myelosuppression
Inform patients that oxaliplatin injection can cause low blood cell counts and the need for frequent monitoring of blood cell counts. Advise patients to contact their healthcare provider immediately for bleeding, fever, particularly if associated with persistent diarrhea, or symptoms of infection develop [see Warnings and Precautions (5.3)].
Posterior Reversible Encephalopathy Syndrome
Advise patients of the potential effects of vision abnormalities, in particular transient vision loss (reversible following therapy discontinuation), which may affect the patients' ability to drive and use machines [see Warnings and Precautions (5.4)].
Pulmonary Toxicity
Advise patients to report immediately to their healthcare provider any persistent or recurrent respiratory symptoms, such as non-productive cough and dyspnea [see Warnings and Precautions (5.5)].
Hepatotoxicity
Advise patients to report signs or symptoms of hepatic toxicity to their healthcare provider [see Warnings and Precautions (5.6)].
QT Interval Prolongation
Advise patients that oxaliplatin injection can cause QTc interval prolongation and to inform their physician if they have any symptoms, such as syncope [see Warnings and Precautions (5.7)].
Rhabdomyolysis
Advise patients to contact their healthcare provider immediately for new or worsening signs or symptoms of muscle toxicity, dark urine, decreased urine output, or the inability to urinate [see Warnings and Precautions (5.8)].
Hemorrhage
Advise patients that oxaliplatin injection may increase the risk of bleeding and to promptly inform their healthcare provider of any bleeding episodes [see Warnings and Precautions (5.9)].
Embryo-Fetal Toxicity
Advise females of reproductive potential of the potential risk to a fetus. Advise females to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.10), Use in Specific Populations (8.1)].
Advise females of reproductive potential to use effective contraception during treatment with oxaliplatin injection and for 9 months after the final dose [see Use in Specific Populations (8.3)].
Advise male patients with female partners of reproductive potential to use effective contraception during treatment with oxaliplatin injection and for 6 months after the final dose [see Use in Specific Populations (8.3), Nonclinical Toxicology (13.1)].
Lactation
Advise women not to breastfeed during treatment with oxaliplatin injection and for 3 months after the final dose [see Use in Specific Populations (8.2)].
Infertility
Advise females and males of reproductive potential that oxaliplatin injection may impair fertility [see Use in Specific Populations (8.3), Nonclinical Toxicology (13.1)].
Gastrointestinal
Advise patients to contact their healthcare provider for persistent vomiting, diarrhea, or signs of dehydration [see Adverse Reactions (6.1)].
Drug Interactions
Inform patients about the risk of drug interactions and the importance of providing a list of prescription and nonprescription drugs to their healthcare provider [see Drug Interactions (7)].
sagent®
Mfd. for SAGENT Pharmaceuticals
Schaumburg, IL 60173 (USA)
Made in China
©2025 Sagent Pharmaceuticals
Revised: May 2025
DESCRIPTION SECTION
11 DESCRIPTION
Oxaliplatin is a platinum-based drug with the molecular formula C8H14N2O4Pt and the chemical name of cis-[(1 R,2 R)-1,2-cyclohexanediamine-N,N′] [oxalato(2-)- O,O′] platinum. Oxaliplatin is an organoplatinum complex in which the platinum atom is complexed with 1,2-diaminocyclohexane (DACH) and with an oxalate ligand as a leaving group.
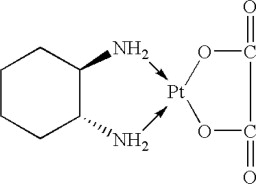
The molecular weight is 397.3. Oxaliplatin is slightly soluble in water at 6 mg/mL, very slightly soluble in methanol, and practically insoluble in ethanol and acetone.
Oxaliplatin Injection, USP, for intravenous use, is supplied in vials containing 50 mg or 100 mg of oxaliplatin as a sterile, preservative-free, aqueous solution at a concentration of 5 mg/mL. Water for Injection, USP is present as an inactive ingredient. Tartaric Acid, NF and Sodium Hydroxide, NF are used in combination as a buffering system.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Adjuvant Treatment with Oxaliplatin in Combination with Fluorouracil
and Leucovorin
The efficacy of oxaliplatin in combination with fluorouracil (FU)/leucovorin (LV) was evaluated in an international, multicenter, randomized (1:1) trial (The Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer [MOSAIC], NCT00275210) in patients with stage II (Dukes' B2) or III (Dukes' C) colon cancer who had undergone complete resection of the primary tumor. Patients were randomized to receive oxaliplatin with fluorouracil/leucovorin or fluorouracil/leucovorin alone for a total of 6 months (i.e., 12 cycles). Table 14 shows the dosing regimens for the two arms.
Eligible patients were between 18 and 75 years of age, had histologically proven stage II (T3-T4 N0 M0; Dukes' B2) or III (any T N1-2 M0; Dukes' C) colon carcinoma (with the inferior pole of the tumor above the peritoneal reflection, i.e., greater than or equal to 15 cm from the anal margin) and had undergone (within 7 weeks prior to randomization) complete resection of the primary tumor without gross or microscopic evidence of residual disease and carcino-embryogenic antigen (CEA) less than 10 ng/mL. Additional eligibility criteria were no prior chemotherapy, immunotherapy or radiotherapy; Eastern Cooperative Oncology Group performance status of 0, 1, or 2 (Karnofsky Performance Status greater than or equal to 60%); no pre-existing neuropathy; and absolute neutrophil count (ANC) greater than or equal to 1.5 x 109/L, platelets greater than or equal to 100 x 109/L, serum creatinine less than or equal to 1.25 x upper limit normal (ULN), total bilirubin less than 2 x ULN, and aspartate transaminase (AST)/alanine transaminase (ALT) less than 2 x ULN. The major efficacy outcome was 3-year disease-free survival (DFS).
Table 14: Dosing Regimens in Adjuvant Treatment Study|
Treatment Arm |
Dose |
Regimen |
|
Oxaliplatin + |
Day 1: Oxaliplatin: 85 mg/m2 (2-hour infusion) + LV: |
every 2 weeks 12 cycles |
|
FU/LV | ||
|
(FOLFOX4) | ||
|
(N =1123) |
Day 2: LV: 200 mg/m2 (2-hour infusion), followed by | |
|
Day 1: LV: 200 mg/m2 (2-hour infusion), followed by |
every 2 weeks | |
|
FU/LV | ||
|
(N=1123) | ||
|
Day 2: LV: 200 mg/m2 (2-hour infusion), followed by | ||
There were 2246 patients enrolled, of whom 1347 (60%) had Stage III disease. Tables 15 and 16 show the baseline characteristics and exposure to oxaliplatin.
Table 15: Baseline Characteristics in Adjuvant Treatment Study|
Oxaliplatin + Infusional |
Infusional FU/LV | |
|
N=1123 |
N=1123 | |
|
Sex: Male (%) |
56.1 |
52.4 |
|
Female (%) |
43.9 |
47.6 |
|
Median age (years) |
61.0 |
60.0 |
|
<65 years of age (%) |
64.4 |
66.2 |
|
≥65 years of age (%) |
35.6 |
33.8 |
|
KPS (%) | ||
|
100 |
29.7 |
30.5 |
|
90 |
52.2 |
53.9 |
|
80 |
4.4 |
3.3 |
|
70 |
13.2 |
11.9 |
|
≤60 |
0.6 |
0.4 |
|
Primary site (%) | ||
|
Colon including cecum |
54.6 |
54.4 |
|
Sigmoid |
31.9 |
33.8 |
|
Recto sigmoid |
12.9 |
10.9 |
|
Other including rectum |
0.6 |
0.9 |
|
Bowel obstruction (%) | ||
|
Yes |
17.9 |
19.3 |
|
Perforation (%) | ||
|
Yes |
6.9 |
6.9 |
|
Stage at Randomization (%) | ||
|
II (T=3,4 N=0, M=0) |
40.1 |
39.9 |
|
III (T=any, N=1,2, M=0) |
59.6 |
59.3 |
|
IV (T=any, N=any, M=1) |
0.4 |
0.8 |
|
Staging - T (%) | ||
|
T1 |
0.5 |
0.7 |
|
T2 |
4.5 |
4.8 |
|
T3 |
76.0 |
75.9 |
|
T4 |
19.0 |
18.5 |
|
Staging - N (%) | ||
|
N0 |
40.2 |
39.9 |
|
N1 |
39.4 |
39.4 |
|
N2 |
20.4 |
20.7 |
|
Staging - M (%) | ||
|
M1 |
0.4 |
0.8 |
|
Oxaliplatin + |
Infusional | |
|
Median Relative Dose Intensity (%) | ||
|
FU |
84.4 |
97.7 |
|
Oxaliplatin |
80.5 |
N/A |
|
Median Number of Cycles |
12 |
12 |
|
Median Number of Cycles with Oxaliplatin |
11 |
N/A |
The median duration of follow-up was approximately 77 months. In the overall and the stage III colon cancer populations, DFS was statistically significantly improved in the oxaliplatin-containing arm compared to fluorouracil/leucovorin alone; however, a statistically significant improvement in DFS was not observed in Stage II patients. No significant differences in overall survival (OS) were detected in the overall population or those with Stage III disease. Table 17 and Figures 1 and 2 summarize the 5-year DFS rates in the overall randomized population and in patients with stage II and III disease based on an intention-to-treat (ITT) analysis.
Table 17: Summary of DFS Analysis in Adjuvant Treatment Study - ITT Population|
A hazard ratio of less than 1 favors Oxaliplatin + Infusional FU/LV | ||
|
Data cut off for disease-free survival June 1, 2006 | ||
|
Parameter |
Oxaliplatin + |
Infusional |
|
Overall | ||
|
Number of patients |
1123 |
1123 |
|
Number of events - relapse or death (%) |
304 (27.1) |
360 (32.1) |
|
5-yr Disease-free survival % (95% CI) |
73.3 (70.7, 76.0) |
67.4 (64.6, 70.2) |
|
Hazard ratio (95% CI) |
0.80 (0.68, 0.93) | |
|
Stratified Log rank test |
p=0.003 | |
|
Stage III (Dukes' C) | ||
|
Number of patients |
672 |
675 |
|
Number of events - relapse or death (%) |
226 (33.6) |
271 (40.1) |
|
5-yr Disease-free survival % (95% CI) |
66.4 (62.7, 70.0) |
58.9 (55.2, 62.7) |
|
Hazard ratio (95% CI) |
0.78 (0.65, 0.93) | |
|
Log rank test |
p=0.005 | |
|
Stage II (Dukes' B2) | ||
|
Number of patients |
451 |
448 |
|
Number of events - relapse or death (%) |
78 (17.3) |
89 (19.9) |
|
5-yr Disease-free survival % (95% CI) |
83.7 (80.2, 87.1) |
79.9 (76.2, 83.7) |
|
Hazard ratio (95% CI) |
0.84 (0.62, 1.14) | |
|
Log rank test |
p=0.258 |
Figure 1: Kaplan-Meier Curves of Disease-Free Survival (cutoff: 1 June 2006) in Adjuvant Treatment Trial – ITT Population
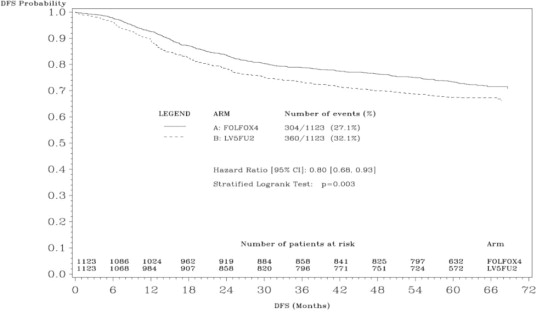
Figure 2: Kaplan-Meier Curves of Disease-Free Survival in Stage III Patients (cutoff: 1 June 2006) in Adjuvant Treatment Trial – ITT Population
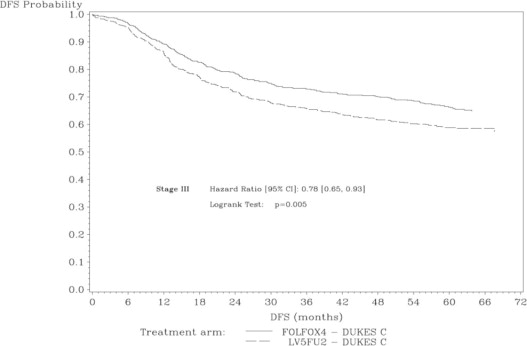
Table 18 summarizes the OS results in the overall randomized population and in patients with stage II and III disease, based on the ITT analysis.
Table 18: Summary of OS Analysis in Adjuvant Treatment - ITT Population|
A hazard ratio of less than 1 favors oxaliplatin + Infusional FU/LV | ||
|
Data cut off for overall survival January 16, 2007 | ||
|
Parameter |
Oxaliplatin + |
Infusional FU/LV |
|
Infusional FU/LV | ||
|
Overall | ||
|
Number of patients |
1123 |
1123 |
|
Number of death events (%) |
245 (21.8) |
283 (25.2) |
|
Hazard ratio (95% CI) |
0.84 (0.71, 1.00) | |
|
Stage III (Dukes' C) | ||
|
Number of patients |
672 |
675 |
|
Number of death events (%) |
182 (27.1) |
220 (32.6) |
|
Hazard ratio (95% CI) |
0.80 (0.65, 0.97) | |
|
Stage II (Dukes' B2) | ||
|
Number of patients |
451 |
448 |
|
Number of death events (%) |
63 (14.0) |
63 (14.1) |
|
Hazard ratio (95% CI) |
1.00 (0.70, 1.41) |
14.2 Previously Untreated Advanced Colorectal Cancer
The efficacy of oxaliplatin in combination with fluorouracil (FU)/leucovorin (LV) was evaluated in a North American, multicenter, open-label, randomized, active-controlled trial (A Randomized Phase III Trial of Three Different Regimens of CPT-11 Plus 5-Fluorouracil and Leucovorin Compared to 5-Fluorouracil and Leucovorin in Patients with Advanced Adenocarcinoma of the Colon and Rectum; NCT00003594). The trial included 7 arms at different times during its conduct, four of which were closed due to either changes in the standard of care, toxicity, or simplification. During the trial, the control arm was changed to irinotecan with fluorouracil/leucovorin.
The results reported below compared the efficacy of oxaliplatin with fluorouracil/leucovorin and oxaliplatin with irinotecan to an approved control regimen of irinotecan with fluorouracil/leucovorin in 795 concurrently randomized patients previously untreated for locally advanced or metastatic colorectal cancer. Table 19 presents the dosing regimens for the three arms. After completion of enrollment, the dose of irinotecan with fluorouracil/leucovorin was decreased due to toxicity.
Eligible patients were at least 18 years of age; had known locally advanced, locally recurrent, or metastatic colorectal adenocarcinoma not curable by surgery or amenable to radiation therapy; with an Eastern Cooperative Oncology Group (ECOG) performance status ≤0, 1, or 2. Patients had to have absolute neutrophil count (ANC) greater than or equal to 1.5 x 109/L, platelets greater than or equal to 100 x 109/L, hemoglobin greater than or equal to 9.0 g/dL, creatinine less than or equal to 1.5 x upper limit of normal (ULN), total bilirubin less than or equal to 1.5 mg/dL, aspartate transaminase (AST) less than or equal to 5 x ULN, and alkaline phosphatase less than or equal to 5 x ULN. Patients may have received adjuvant treatment for resected Stage II or III disease without recurrence within 12 months. Randomization was stratified by ECOG performance status (0, 1 vs 2), prior adjuvant chemotherapy (yes vs no), prior immunotherapy (yes vs no), and age (less than 65 vs greater than or equal to 65 years). Although no post study treatment was specified in the protocol, 65% to 72% of patients received additional post study chemotherapy after study treatment discontinuation on all arms. Fifty-eight percent of patients on the oxaliplatin with fluorouracil/leucovorin arm received an irinotecan-containing regimen and 23% of patients on the irinotecan with fluorouracil/leucovorin arm received an oxaliplatin-containing regimen.
The main efficacy outcome measure was 3-year disease-free survival (DFS) and additional efficacy outcome measures were overall survival (OS).
Table 19: Dosing Regimens for Previously Untreated Advanced Colorectal Cancer Clinical Trial|
Treatment Arm |
Dose |
Regimen |
|
Oxaliplatin + |
Day 1: Oxaliplatin: 85 mg/m2 (2-hour infusion) + LV 200 mg/m2 (2-hour
infusion), followed by Day 2: LV 200 mg/m2 (2-hour infusion), followed by |
every 2 weeks |
|
Irinotecan + |
Day 1: Irinotecan 125 mg/m2 as a 90–min infusion + LV 20 mg/m2 |
every 6 weeks |
|
as a 15-min infusion or intravenous push, followed by | ||
|
FU 500 mg/m2 intravenous bolus weekly x 4 | ||
|
Oxaliplatin + |
Day 1: Oxaliplatin: 85 mg/m2 intravenous (2-hour infusion) + irinotecan 200 mg/m2 intravenous over 30 minutes |
every 3 weeks |
Table 20 presents the baseline characteristics.
Table 20: Baseline Characteristics for Previously Untreated Advanced Colorectal Cancer Clinical Trial|
Oxaliplatin + |
Irinotecan + |
Oxaliplatin + | |
|
FU/LV |
FU/LV |
Irinotecan | |
|
N=267 |
N=264 |
N=264 | |
|
Sex: Male (%) |
58.8 |
65.2 |
61.0 |
|
Female (%) |
41.2 |
34.8 |
39.0 |
|
Median age (years) |
61.0 |
61.0 |
61.0 |
|
<65 years of age (%) |
61 |
62 |
63 |
|
≥65 years of age (%) |
39 |
38 |
37 |
|
ECOG (%) | |||
|
0-1 |
94.4 |
95.5 |
94.7 |
|
2 |
5.6 |
4.5 |
5.3 |
|
Involved organs (%) | |||
|
Colon only |
0.7 |
0.8 |
0.4 |
|
Liver only |
39.3 |
44.3 |
39.0 |
|
Liver + other |
41.2 |
38.6 |
40.9 |
|
Lung only |
6.4 |
3.8 |
5.3 |
|
Other (including lymph nodes) |
11.6 |
11.0 |
12.9 |
|
Not reported |
0.7 |
1.5 |
1.5 |
|
Prior radiation (%) |
3.0 |
1.5 |
3.0 |
|
Prior surgery (%) |
74.5 |
79.2 |
81.8 |
|
Prior adjuvant (%) |
15.7 |
14.8 |
15.2 |
The median number of cycles administered per patient was 10 (23.9 weeks) for the oxaliplatin plus fluorouracil/leucovorin regimen, 4 (23.6 weeks) for the irinotecan plus fluorouracil/leucovorin regimen, and 7 (21.0 weeks) for the oxaliplatin plus irinotecan regimen.
Patients who received oxaliplatin with fluorouracil/leucovorin had a significantly longer time to tumor progression based on investigator assessment, longer OS, and a significantly higher confirmed response rate based on investigator assessment compared to patients who received irinotecan with fluorouracil/leucovorin. Efficacy results are summarized in Table 21 and Figure 3.
Table 21: Efficacy Results for Previously Untreated Advanced Colorectal Cancer Trial|
*A hazard ratio of less than 1 favors Oxaliplatin + Infusional fluorouracil/leucovorin | |||
|
†Compared to irinotecan plus fluorouracil/leucovorin (IFL) arm | |||
|
‡ Based on all patients with measurable disease at baseline | |||
|
The numbers in the response rate and TTP analysis are based on unblinded investigator assessment. | |||
|
Oxaliplatin + |
Irinotecan + |
Oxaliplatin + | |
|
FU/LV |
Irinotecan | ||
|
N=264 |
N=264 | ||
|
Survival (ITT) | |||
|
Number of deaths (%) |
155 (58.1) |
192 (72.7) |
175 (66.3) |
|
Median survival (months) |
19.4 |
14.6 |
17.6 |
|
Hazard ratio (95% CI)* |
0.65 (0.53, 0.80)† |
- | |
|
P-value |
<0.0001† |
- | |
|
TTP (ITT, investigator assessment) | |||
|
Percentage of progressors |
82.8 |
81.8 |
89.4 |
|
Median TTP (months) |
8.7 |
6.9 |
6.5 |
|
Hazard ratio (95% CI)* |
0.74 (0.61, 0.89)† |
- | |
|
P-value |
0.0014† |
- | |
|
Response Rate (investigator assessment)‡ | |||
|
Patients with measurable disease |
210 |
212 |
215 |
|
Complete response, N (%) |
13 (6.2) |
5 (2.4) |
7 (3.3) |
|
Partial response, N (%) |
82 (39.0) |
64 (30.2) |
67 (31.2) |
|
Complete and partial response, N (%) |
95 (45.2) |
69 (32.5) |
74 (34.4) |
|
95% CI |
(38.5, 52.0) |
(26.2, 38.9) |
(28.1, 40.8) |
|
P-value |
0.0080† |
- |
Figure 3: Kaplan-Meier Curves for Overall Survival in Previously Untreated Advanced Colorectal Cancer Trial
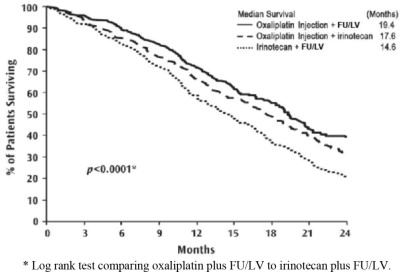
- Log rank test comparing oxaliplatin plus FU/LV to irinotecan plus FU/LV.
In descriptive subgroup analyses, the improvement in overall survival (OS) for oxaliplatin with fluorouracil/leucovorin compared to irinotecan with fluorouracil/leucovorin appeared to be maintained across age groups, prior adjuvant treatment, number of organs involved and both sexes; however, the effect appeared larger among women than men.
14.3 Previously Treated Advanced Colorectal Cancer
The efficacy of oxaliplatin in combination with fluorouracil (FU)/leucovorin (LV) was evaluated in a multicenter, open-label, randomized, three-arm controlled trial was conducted in the US and Canada in patients with advanced colorectal cancer who had relapsed/progressed during or within 6 months of first-line treatment with bolus fluorouracil/leucovorin and irinotecan (A multicenter, open-label, randomized, three-arm study of 5-fluorouracil (5-FU)
- leucovorin (LV) or oxaliplatin or a combination of 5-FU/LV + oxaliplatin as second-line treatment of metastatic colorectal carcinoma: NCT00008281). Patients were randomized to one of three regimens; the dosing regimens are presented in Table 22. Eligible patients were at least 18 years of age, had unresectable, measurable, histologically proven colorectal adenocarcinoma, with a Karnofsky performance status (KPS) greater than 50%. Patients had to have aspartate transaminase (AST), alanine transaminase (ALT) and alkaline phosphatase less than or equal to 2x upper limit of normal (ULN), unless liver metastases were present and documented at baseline by CT or MRI scan, in which case less than or equal to 5 x ULN was permitted. Prior radiotherapy was permitted if it had been completed at least 3 weeks before randomization. The main efficacy outcome measure was 3-year disease-free survival (DFS) and an additional outcome measure was overall survival (OS).
|
Treatment Arm |
Dose |
Regimen |
|
Oxaliplatin + |
Day 1: Oxaliplatin: 85 mg/m2 (2-hour infusion) + LV 200 mg/m2 (2-hour
infusion), followed by Day 2: LV 200 mg/m2 (2-hour infusion), followed by |
every 2 weeks |
|
FU/LV |
Day 1: LV 200 mg/m2 (2-hour infusion), followed by Day 2: LV 200 mg/m2 (2-hour infusion), followed by |
every 2 weeks |
|
Oxaliplatin |
Day 1: Oxaliplatin 85 mg/m2 (2-hour infusion) |
every 2 weeks |
Patients must have had at least one unidimensional lesion measuring greater than or equal to 20 mm using conventional CT or MRI scans or greater than or equal to 10 mm using a spiral CT scan. Tumor response and progression were assessed every 3 cycles (6 weeks) using the Response Evaluation Criteria in Solid Tumors (RECIST) until radiological documentation of progression or for 13 months following the first dose of study drug(s), whichever came first. Confirmed responses were based on two tumor assessments separated by at least 4 weeks. Baseline characteristics are shown in Table 23.
Table 23: Baseline Characteristics in Refractory and Relapsed Colorectal Cancer Trial|
Oxaliplatin + |
Oxaliplatin |
FU/LV | |
|
FU/LV |
N=156 |
N=151 | |
|
N=152 | |||
|
Sex: Male (%) |
57.2 |
60.9 |
54.3 |
|
Female (%) |
42.8 |
39.1 |
45.7 |
|
Median age (years) |
59.0 |
61.0 |
60.0 |
|
Range |
22 to 88 |
27 to 79 |
21 to 80 |
|
Race (%) | |||
|
Caucasian |
88.8 |
84.6 |
87.4 |
|
Black |
5.9 |
7.1 |
7.9 |
|
Asian |
2.6 |
2.6 |
1.3 |
|
Other |
2.6 |
5.8 |
3.3 |
|
KPS (%) | |||
|
70 to 100 |
95.4 |
92.3 |
94.7 |
|
50 to 60 |
2.0 |
4.5 |
2.6 |
|
Not reported |
2.6 |
3.2 |
2.6 |
|
Prior radiotherapy (%) |
25.0 |
19.2 |
25.2 |
|
Prior pelvic radiation (%) |
21.1 |
13.5 |
18.5 |
|
Number of metastatic sites (%) | |||
|
1 |
25.7 |
31.4 |
27.2 |
|
≥2 |
74.3 |
67.9 |
72.2 |
|
Liver involvement (%) | |||
|
Liver only |
18.4 |
25.6 |
22.5 |
|
Liver + other |
53.3 |
59.0 |
60.3 |
The median number of cycles administered per patient was 6 for the oxaliplatin and fluorouracil/leucovorin combination and 3 each for fluorouracil/leucovorin alone and oxaliplatin alone. Patients treated with the combination of oxaliplatin and fluorouracil/leucovorin had an increased response rate compared to patients given fluorouracil/leucovorin or oxaliplatin alone. Efficacy results are summarized in Tables 24 and 25.
Table 24: Response Rates in Refractory and Relapsed Colorectal Cancer Clinical Trial - ITT Analysis|
Best Response |
Oxaliplatin + |
Oxaliplatin |
FU/LV |
|
FU/LV |
N=156 |
N=151 | |
|
N=152 | |||
|
Complete Response |
0 |
0 |
0 |
|
Partial Response |
13 (9%) |
2 (1%) |
0 |
|
P-value |
0.0002 FU/LV vs Oxaliplatin + FU/LV | ||
|
95% CI |
4.6%, 14.2% |
0.2%, 4.6% |
0, 2.4% |
|
*This is not an ITT analysis. Events were limited to radiographic disease progression documented by independent review of radiographs. Clinical progression was not included in this analysis, and 18% of patients were excluded from the analysis based on unavailability of the radiographs for independent review. | |||
|
Arm |
Oxaliplatin + |
Oxaliplatin |
FU/LV |
|
FU/LV |
N=156 |
N=151 | |
|
N=152 | |||
|
Number of progressors |
50 |
101 |
74 |
|
Number of patients with no radiological evaluation beyond baseline |
17 (11%) |
16 (10%) |
22 (15%) |
|
Median TTP (months) |
4.6 |
1.6 |
2.7 |
|
95% CI |
4.2, 6.1 |
1.4, 2.7 |
1.8, 3.0 |
At the time of the interim analysis 49% of the radiographic progression events had occurred. In this interim analysis an estimated 2-month increase in median time to radiographic progression was observed compared to fluorouracil/leucovorin alone.
