Allergy Relief
Equate 44-614 Delisted
ba0fa9f9-7778-4a51-9ad1-fb349ceb8121
HUMAN OTC DRUG LABEL
May 28, 2025
Wal-Mart Stores Inc
DUNS: 051957769
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Diphenhydramine HCI
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Drug Labeling Information
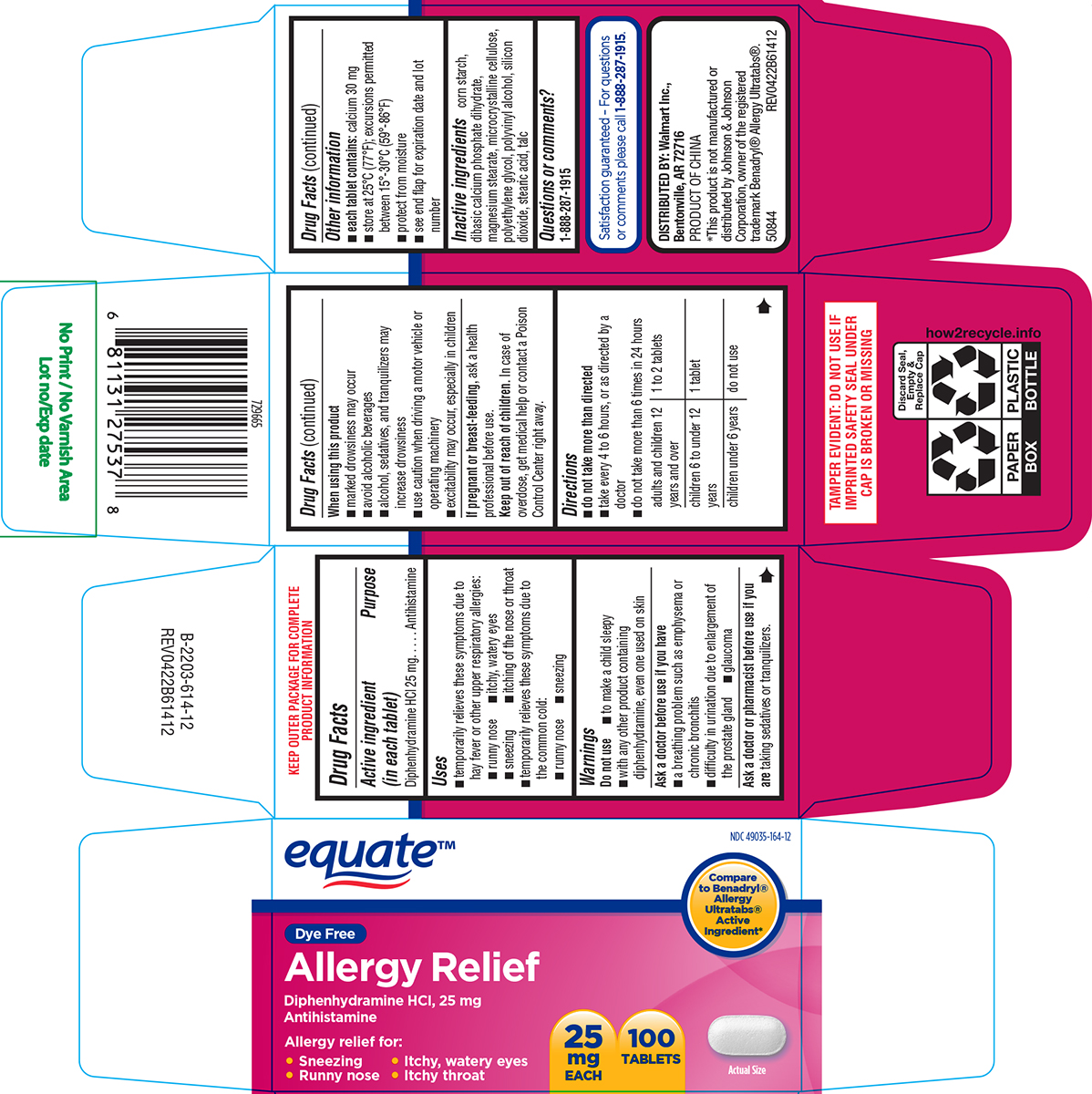
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal display panel
equate™
NDC 49035-164-12
Compare to Benadryl® Allergy Ultratabs® Active Ingredient*
Dye Free
Allergy Relief
Diphenhydramine HCI, 25 mg
Antihistamine
Allergy relief for:
• Sneezing • Itchy, watery eyes
• Runny nose • Itchy throat
25
mg
EACH
100
TABLETS
Actual Size
TAMPER EVIDENT: DO NOT USE IF
IMPRINTED SAFETY SEAL UNDER
CAP IS BROKEN OR MISSING
Satisfaction guaranteed – For questions
or comments please call1-888-287-1915.
DISTRIBUTED BY: Walmart Inc.,
Bentonville, AR 72716
PRODUCT OF CHINA
*This product is not manufactured or
distributed by Johnson & Johnson
Corporation, owner of the registered
trademark Benadryl® Allergy Ultratabs®.
50844 REV0422B61412
Equate 44-614
INDICATIONS & USAGE SECTION
Uses
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- itchy, watery eyes
- sneezing
- itching of the nose or throat
- temporarily relieves these symptoms due to the common cold:
- runny nose
- sneezing
OTC - ACTIVE INGREDIENT SECTION
Active ingredient (in each tablet)
Diphenhydramine HCl 25 mg
OTC - PURPOSE SECTION
Purpose
Antihistamine
WARNINGS SECTION
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- difficulty in urination due to enlargement of the prostate gland
- glaucoma
Ask a doctor or pharmacist before use if you are
taking sedatives or tranquilizers.
When using this product
- marked drowsiness may occur
- avoid alcoholic beverages
- alcohol, sedatives, and tranquilizers may increase drowsiness
- use caution when driving a motor vehicle or operating machinery
- excitability may occur, especially in children
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
Directions
*do not take more than directed
- take every 4 to 6 hours, or as directed by a doctor
- do not take more than 6 times in 24 hours
|
adults and children 12 years and over |
1 to 2 tablets |
|
children 6 to under 12 years |
1 tablet |
|
children under 6 years |
do not use |
STORAGE AND HANDLING SECTION
Other information
***each tablet contains:**calcium 30 mg
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- protect from moisture
- see end flap for expiration date and lot number
INACTIVE INGREDIENT SECTION
Inactive ingredients
corn starch, dibasic calcium phosphate dihydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, silicon dioxide, stearic acid, talc
OTC - QUESTIONS SECTION
Questions or comments?
1-888-287-1915
