Gentamicin Sulfate
Gentamicin Sulfate Ophthalmic Solution, USP 0.3% (Sterile) Rx only
f6fb1949-a89b-4fe0-afdc-31ca6c02e567
HUMAN PRESCRIPTION DRUG LABEL
Jul 30, 2025
REMEDYREPACK INC.
DUNS: 829572556
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Gentamicin Sulfate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
DRUG: Gentamicin Sulfate
GENERIC: Gentamicin Sulfate
DOSAGE: SOLUTION/ DROPS
ADMINSTRATION: OPHTHALMIC
NDC: 70518-3785-0
PACKAGING: 5 mL in 1 BOTTLE, DROPPER
OUTER PACKAGING: 1 in 1 CARTON
ACTIVE INGREDIENT(S):
- GENTAMICIN SULFATE 3mg in 1mL
INACTIVE INGREDIENT(S):
- SODIUM CHLORIDE
- SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM
- WATER
- HYDROCHLORIC ACID
- SODIUM HYDROXIDE
- BENZALKONIUM CHLORIDE
- SODIUM PHOSPHATE, DIBASIC, UNSPECIFIED FORM

INDICATIONS & USAGE SECTION
INDICATIONS AND USAGE
Gentamicin sulfate ophthalmic solution is indicated in the topical treatment of ocular bacterial infections including conjunctivitis, keratitis, keratoconjunctivitis, corneal ulcers, blepharitis, blepharoconjunctivitis, acute meibomianitis, and dacryocystitis, caused by susceptible strains of the following microorganisms:
Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pyogenes, Streptococcus pneumoniae, Enterobacter aerogenes, Escherichia coli, Haemophilus influenzae, Klebsiella pneumoniae, Neisseria gonorrhoeae, Pseudomonas aeruginosa,and Serratia marcescens.
CONTRAINDICATIONS SECTION
CONTRAINDICATIONS
Gentamicin sulfate ophthalmic solution is contraindicated in patients with known hypersensitivity to any of its components.
ADVERSE REACTIONS SECTION
ADVERSE REACTIONS
Bacterial and fungal corneal ulcers have developed during treatment with gentamicin ophthalmic preparations.
The most frequently reported adverse reactions are ocular burning and irritation upon drug instillation, non-specific conjunctivitis, conjunctival epithelial defects and conjunctival hyperemia.
Other adverse reactions which have occurred rarely are allergic reactions, thrombocytopenic purpura and hallucinations.
To report SUSPECTED ADVERSE REACTIONS, contact Bausch & Lomb Incorporated at 1-800-553-5340 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DOSAGE & ADMINISTRATION SECTION
DOSAGE AND ADMINISTRATION
Instill one or two drops into the affected eye(s) every four hours. In severe infections dosage may be increased to as much as two drops every hour.
WARNINGS SECTION
WARNINGS
NOT FOR INJECTION INTO THE EYE
Gentamicin sulfate ophthalmic solution is not for injection. It should never be injected subconjunctivally, nor should it be directly introduced into the anterior chamber of the eye.
CLINICAL PHARMACOLOGY SECTION
CLINICAL PHARMACOLOGY
Microbiology
Gentamicin sulfate is active in vitroagainst many strains of the following microorganisms:
Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pyogenes, Streptococcus pneumoniae, Enterobacter aerogenes, Escherichia coli, Haemophilus influenzae, Klebsiella pneumoniae, Neisseria gonorrhoeae, Pseudomonas aeruginosa,and Serratia marcescens.
DESCRIPTION SECTION
DESCRIPTION
Gentamicin sulfate ophthalmic solution, USP is a sterile, aqueous solution buffered to approximately pH 7.0 and formulated for ophthalmic use.
Each mL contains:
**Active:**gentamicin sulfate, USP (equivalent to 3 mg gentamicin).
**Inactives:**dibasic sodium phosphate, sodium chloride, monobasic sodium phosphate, purified water. Hydrochloric acid and/or sodium hydroxide may be added to adjust pH (6.5 - 7.5).
**Preservative:**benzalkonium chloride 0.01%.
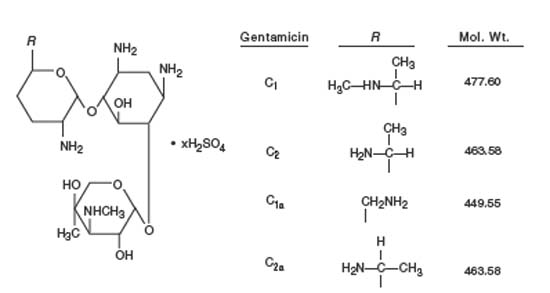
Gentamicin is an aminoglycoside antibiotic obtained from cultures of Micromonospora purpurea. It is a mixture of the sulfate salts of gentamicin C1, C2, C1a and C2a. All three components appear to have similar antimicrobial activity. Gentamicin sulfate occurs as a white to buff powder and is soluble in water and insoluble in alcohol.
The structural formula is as follows:
PRECAUTIONS SECTION
PRECAUTIONS
General
Prolonged use of topical antibiotics may give rise to overgrowth of nonsusceptible organisms including fungi. Bacterial resistance to gentamicin may also develop. If purulent discharge, inflammation or pain becomes aggravated, the patient should discontinue use of the medication and consult a physician. If irritation or hypersensitivity to any component of the drug develops, the patient should discontinue use of this preparation and appropriate therapy should be instituted.
Information for Patients
To avoid contamination, do not touch tip of container to the eye, eyelid or any surface.
Repackaged By / Distributed By: RemedyRepack Inc.
625 Kolter Drive, Indiana, PA 15701
(724) 465-8762
Carcinogenesis, Mutagenesis, Impairment of Fertility
There are no published carcinogenicity or impairment of fertility studies on gentamicin. Aminoglycoside antibiotics have been found to be non-mutagenic.
Pregnancy
Gentamicin has been shown to depress body weights, kidney weights and median glomerular counts in newborn rats when administered systemically to pregnant rats in daily doses approximately 500 times the maximum recommended ophthalmic human dose. There are no adequate and well-controlled studies in pregnant women. Gentamicin should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
HOW SUPPLIED SECTION
HOW SUPPLIED
Gentamicin sulfate ophthalmic solution USP, 0.3% is supplied sterile in a white LDPE plastic bottle, with a white LLDPE extended controlled drop tip and white polypropylene cap in the following size:
NDC: 70518-3785-00
PACKAGING: 1 in 1 CARTON, 5 mL in 1 BOTTLE, DROPPER TYPE 0

NOT FOR INJECTION INTO THE EYE
FOR OPHTHALMIC USE ONLY
Storage:Store between 2°C to 25°C (36°F to 77°F). Avoid exposure to excessive heat.
KEEP OUT OF REACH OF CHILDREN.
Repackaged and Distributed By:
Remedy Repack, Inc.
625 Kolter Dr. Suite #4 Indiana, PA 1-724-465-8762
