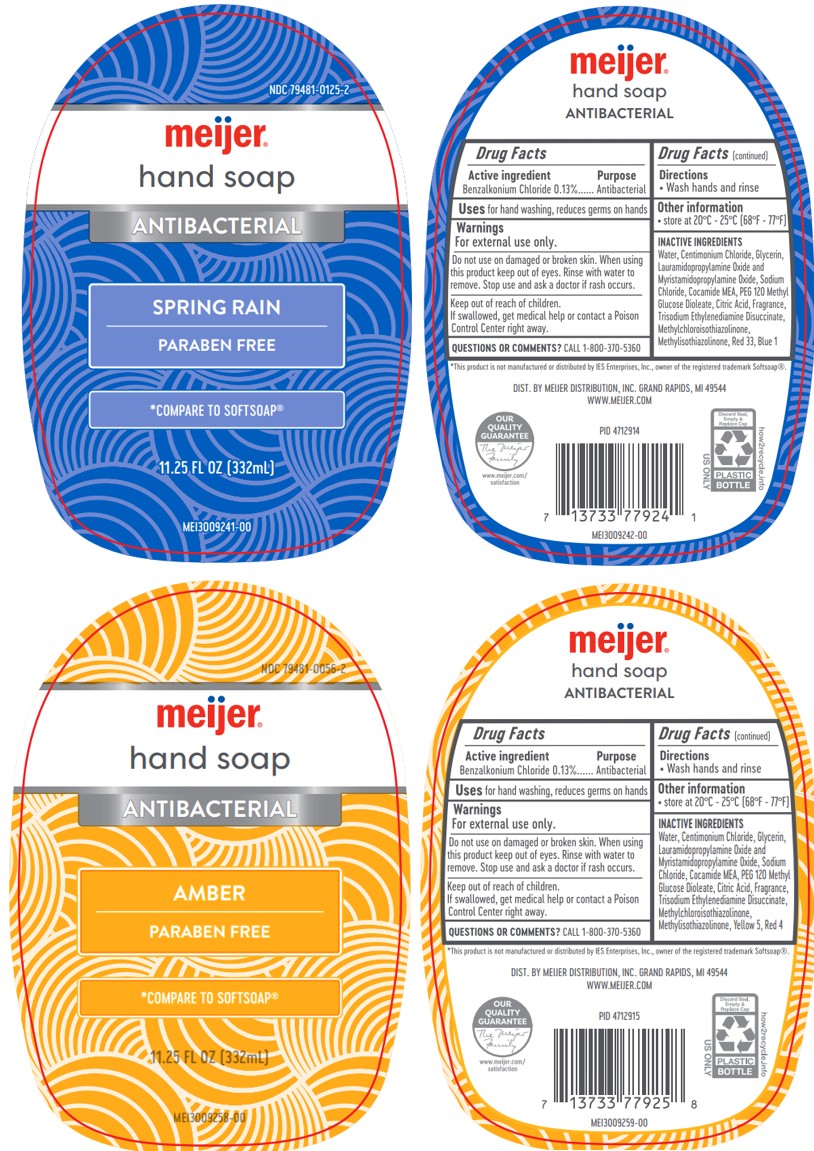
Meijer Antibacterial Spring Rain Hand
Meijer Antibacterial Hand Soap
35bbdd67-6d15-26b6-e063-6394a90a3f96
HUMAN OTC DRUG LABEL
Dec 5, 2024
Meijer, Inc.
DUNS: 006959555
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Benzalkonium Chloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (15)
Benzalkonium Chloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (15)
Benzalkonium Chloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (15)
Drug Labeling Information
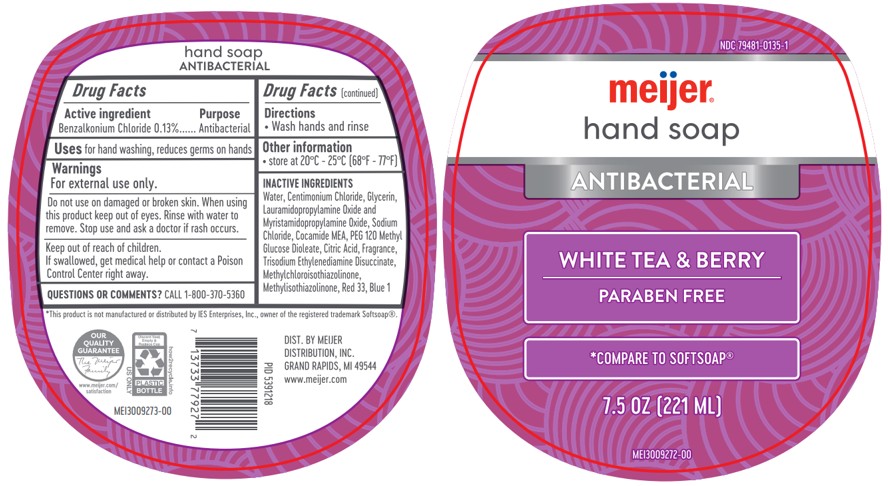
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Meijer Antibacterial Hand Soap
Meijer Antibacterial Hand Soap
11.25 FL OZ
Refill Hand Soap

INDICATIONS & USAGE SECTION
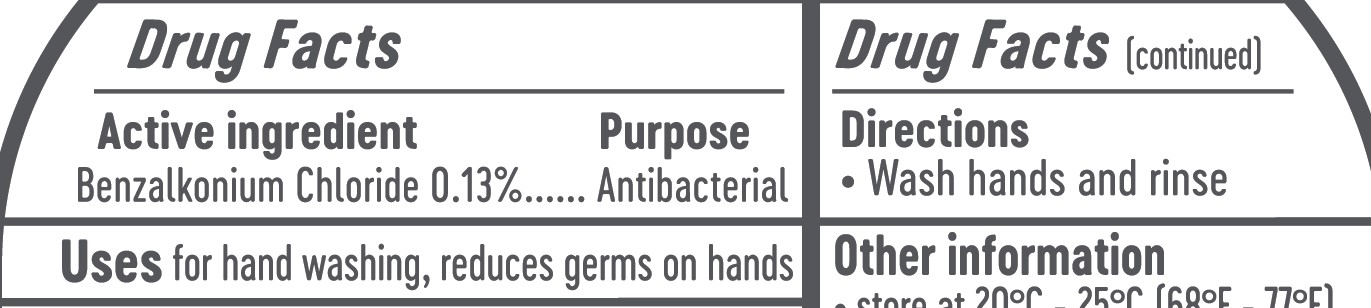
Directions
Wash hands and rinse
WARNINGS SECTION
Warnings
For external use only
Do not use on damaged or broken skin. When using this product keep out of eyes. Rinse with water to remove. Stop use and ask a doctor if rash occurs.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
OTC - ACTIVE INGREDIENT SECTION
Activce Ingredient
Active Ingredient Purpose
Benzalkonium Chloride 0.13%......Antibacterial
OTC - PURPOSE SECTION
Antibacterial
Antibacterial
OTC - QUESTIONS SECTION
Adverse event reporting
QUESTIONS OR COMMENTS? CALL 1-800-370-5360
DOSAGE & ADMINISTRATION SECTION
Dosage and Administration
Apply 1-2 pumps to hands rub and rinse to reduce the amount of bacteria on hands
INACTIVE INGREDIENT SECTION
Inactive Ingredients
Water, Centimonium Chloride, Glycerin, Lauramidopropylamine Oxide and Myristamidopropylamine Oxide, Sodium Chloride, Cocamide MEA, PEG 120 Methyl Glucose Dioleate, Citric Acid, Fragrance, Trisodium Ethylenediamine Disuccinate, Methylchloroisothiazolinone, Methylisothiazolinone
Amber: Yellow 5, Red 4
Spring Rain: Red 33, Blue 1
White Tea and Berry: Red 33, Blue 1
STORAGE AND HANDLING SECTION
Other information: Storage
Avoid extreme temperatures: store at 20-25C (68-77F)
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children
If swallowed, get medical help or contact a Poison Contol Center right away

