ivermectin
Ivermectin Cream
Approved
Approval ID
22e85f9a-0805-47f2-8709-aa6514511b04
Product Type
HUMAN PRESCRIPTION DRUG LABEL
Effective Date
Jun 7, 2023
Manufacturers
FDA
Zydus Lifesciences Limited
DUNS: 918596198
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
ivermectin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
NDC Product Code70771-1728
Application NumberANDA215210
Product Classification
M
Marketing Category
C73584
G
Generic Name
ivermectin
Product Specifications
Route of AdministrationTOPICAL
Effective DateJune 7, 2023
FDA Product Classification
INGREDIENTS (16)
CARBOMER COPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED)Inactive
Code: 809Y72KV36
Classification: IACT
CITRIC ACID MONOHYDRATEInactive
Code: 2968PHW8QP
Classification: IACT
CETYL ALCOHOLInactive
Code: 936JST6JCN
Classification: IACT
IVERMECTINActive
Quantity: 10 mg in 1 g
Code: 8883YP2R6D
Classification: ACTIB
COCO DIETHANOLAMIDEInactive
Code: 92005F972D
Classification: IACT
DIMETHICONEInactive
Code: 92RU3N3Y1O
Classification: IACT
EDETATE DISODIUMInactive
Code: 7FLD91C86K
Classification: IACT
DIMETHYL ISOSORBIDEInactive
Code: SA6A6V432S
Classification: IACT
GLYCERINInactive
Code: PDC6A3C0OX
Classification: IACT
ISOPROPYL PALMITATEInactive
Code: 8CRQ2TH63M
Classification: IACT
SODIUM HYDROXIDEInactive
Code: 55X04QC32I
Classification: IACT
METHYLPARABENInactive
Code: A2I8C7HI9T
Classification: IACT
PROPYLPARABENInactive
Code: Z8IX2SC1OH
Classification: IACT
SODIUM LAURYL SULFATEInactive
Code: 368GB5141J
Classification: IACT
STEARYL ALCOHOLInactive
Code: 2KR89I4H1Y
Classification: IACT
WATERInactive
Code: 059QF0KO0R
Classification: IACT
Drug Labeling Information
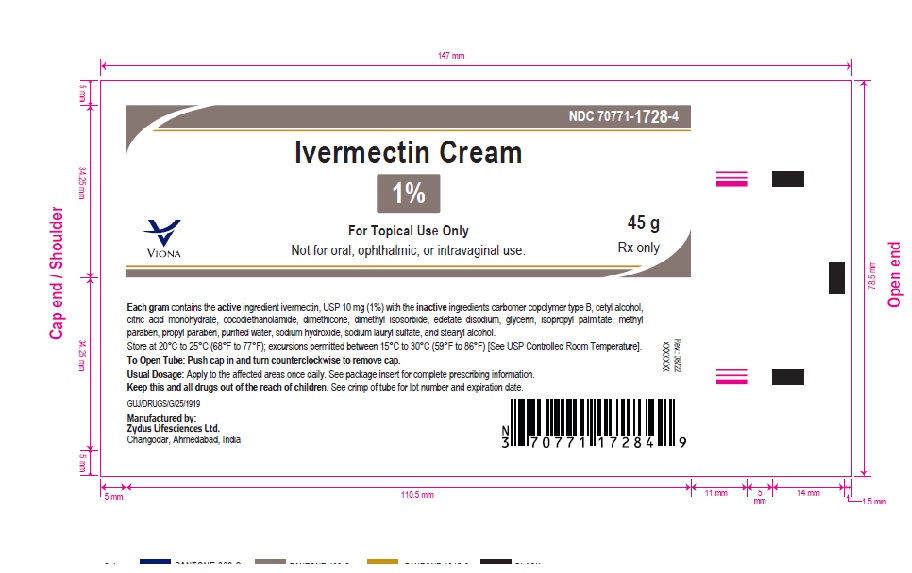
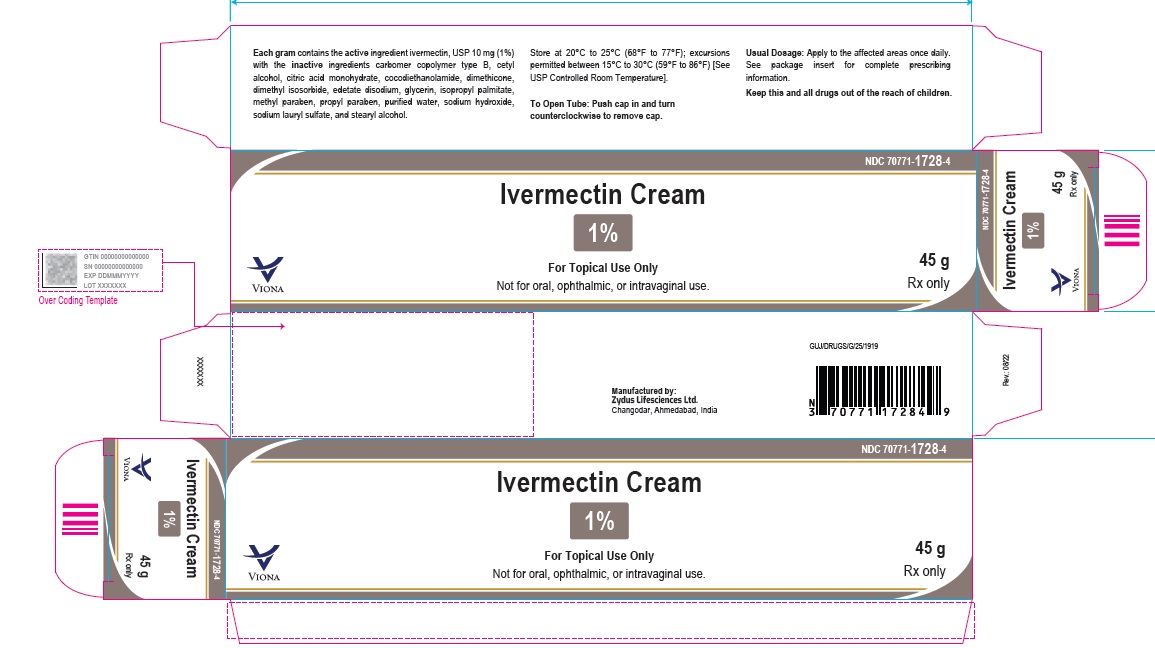
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
LOINC: 51945-4Updated: 8/4/2022
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Ivermectin Cream, 1%
45 gm
NDC 70771-1728-4
Rx only