APIS MELLIFERA
Sevene USA (as PLD) - APIS MELLIFICA 30C (76472-1125)
44713715-fea5-4c14-87c2-1373968474a5
HUMAN OTC DRUG LABEL
May 22, 2025
SEVENE USA
DUNS: 969332936
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
APIS MELLIFERA
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (2)
Drug Labeling Information
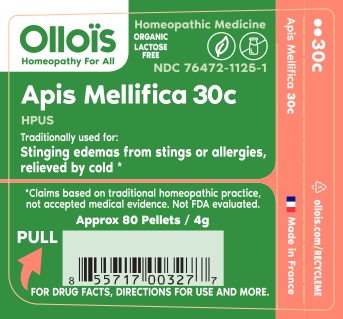
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

INDICATIONS & USAGE SECTION
USE
Condition listed above or as directed by a physician.
DOSAGE & ADMINISTRATION SECTION
DIRECTIONS
(adults/children) Dissolve 5 pellets under the tongue 3 times a day until symptoms are relieved or as directed by a physician.
WARNINGS SECTION
WARNINGS
Stop use and ask a physician if symptoms persist for more than 3 days or worsen.
OTC - PREGNANCY OR BREAST FEEDING SECTION
If pregnant or breast-feeding, ask a health professional before use.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children.
OTHER SAFETY INFORMATION
OTHER INFORMATION
Store at room temperature.
OTC - DO NOT USE SECTION
Do not use if pellet-dispenser seal is broken.
INACTIVE INGREDIENT SECTION
INACTIVE INGREDIENT
Sucrose.
OTC - QUESTIONS SECTION
QUESTIONS?
INFO@OLLOIS.COM * www.ollois.com * MADE IN FRANCE. NOT REVIEWED BY THE FDA AND NOT GUARANTEED TO BE EFFECTIVE. THIS HOMEOPATHIC DILUTION MAY NOT BE SUSCEPTIBLE TO SCIENTIFIC MEASUREMENT.
OTC - PURPOSE SECTION
TRADITIONALLY USED FOR
Stinging edemas from stings or allergies, relieved by cold*
OTC - ACTIVE INGREDIENT SECTION
ACTIVE INGREDIENT
HOMEOPATHIC DILUTION OF HPUS Apis Mellifica 30C.
