Mirtazapine
Mirtazapine Tablets, USP Rx Only These highlights do not include all the information needed to use MIRTAZAPINE TABLETS safely and effectively. See full prescribing information for MIRTAZAPINE TABLETS. Initial U.S. Approval: 1996
e5478a6a-a29a-9f61-e053-2995a90a11d0
HUMAN PRESCRIPTION DRUG LABEL
Sep 23, 2025
AvKARE
DUNS: 796560394
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Mirtazapine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL-7.5mg


INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Suicidal Thoughts and Behaviors
Advise patients and caregivers to look for the emergence of suicidality, especially early during treatment and when the dosage is adjusted up or down, and instruct them to report such symptoms to the healthcare provider [see Boxed Warning and Warnings and Precautions (5.1)].
Agranulocytosis
Advise patients to contact their physician if they experience fever, chills, sore throat, mucous membrane ulceration, flu-like complaints, or other symptoms that might suggest infection [see Warnings and Precautions (5.2)].
Serotonin Syndrome
Caution patients about the risk of serotonin syndrome, particularly with the concomitant use of mirtazapine tablets with other serotonergic drugs including triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, amphetamines, St. John’s Wort, and with drugs that impair metabolism of serotonin (in particular, MAOIs, both those intended to treat psychiatric disorders and also others, such as linezolid). Advise patients to contact their healthcare provider or report to the emergency room if they experience signs or symptoms of serotonin syndrome [see Dosage and Administration (2.4),Contraindications (4),Warnings and Precautions (5.3),Drug Interactions (7)].
QT Prolongation and Torsades de Pointes
Inform patients to consult their physician immediately if they feel faint, lose consciousness, or have heart palpitations [see Warnings and Precautions (5.5),Drug Interactions (7),Overdosage (10)].Advise patients to inform physicians that they are taking mirtazapine tablets before any new drug is taken.
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)
Advise patients to report to their healthcare provider at the earliest onset of fever, rash, swollen lymph nodes, or other signs and symptoms suggestive of Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) [ see Contraindications (4), Warnings and Precautions (5.6)].
Somnolence
Advise patients that mirtazapine tablets may impair judgment, thinking, and particularly, motor skills, because of its prominent sedative effect. Caution patients about performing activities requiring mental alertness, such as operating hazardous machinery or operating a motor vehicle, until they are reasonably certain that mirtazapine tablets therapy does not adversely affect their ability to engage in such activities. [see Warnings and Precautions (5.8)].
Alcohol
Advise patients to avoid alcohol while taking mirtazapine tablets [see Warnings and Precautions (5.8),Drug Interactions (7)].
Activation of Mania/Hypomania
Advise patients and their caregivers to observe for signs of activation of mania/hypomania and instruct them to report such symptoms to the healthcare provider [see Warnings and Precautions (5.9)].
Discontinuation Syndrome
Advise patients not to abruptly discontinue mirtazapine tablets and to discuss any tapering regimen with their healthcare provider. Adverse reactions can occur when mirtazapine tablets are discontinued [see Dosage and Administration (2.6),Warnings and Precautions (5.14)].
Allergic Reactions
Advise patients to notify their healthcare provider if they develop an allergic reaction such as rash, hives, swelling, or difficulty breathing [see Contraindications (4),Adverse Reactions (6.2)].
Pregnancy
- Advise patients to notify their physician if they become pregnant or intend to become pregnant during mirtazapine tablets therapy.
- Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to mirtazapine tablets during pregnancy [see Use in Specific Populations (8.1)].
Lactation
Advise patients to notify their physician if they are breastfeeding an infant [see Use in Specific Populations (8.2)].
Angle-Closure Glaucoma
Patients should be advised that taking mirtazapine tablets can cause mild pupillary dilation, which in susceptible individuals, can lead to an episode of angle-closure glaucoma. Pre-existing glaucoma is almost always open-angle glaucoma because angle-closure glaucoma, when diagnosed, can be treated definitively with iridectomy. Open-angle glaucoma is not a risk factor for angle-closure glaucoma. Patients may wish to be examined to determine whether they are susceptible to angle closure, and have a prophylactic procedure (e.g., iridectomy), if they are susceptible [see Warnings and Precautions (5.4)].
Manufactured for:
AvKARE
Pulaski, TN 38478
Mfg. Rev. 11/21
AV Rev. 09/25 (M)
SPL MEDGUIDE SECTION
MEDICATION GUIDE
Mirtazapine (mir taz’ a peen) Tablets, USP
What is the most important information I should know about mirtazapine tablets?
Mirtazapine tablets may cause serious side effects, including:
***Increased risk of suicidal thoughts or actions in some children and young adults.**Mirtazapine tablets, and other antidepressant medicines may increase suicidal thoughts or actions in some people 24 years of age and younger,especially within the first few months of treatment or when the dose is changed. Mirtazapine tablets are not for use in children. *Depression or other serious mental illnesses are the most important causes of suicidal thoughts or actions.
How can I watch for and try to prevent suicidal thoughts and actions?
- Pay close attention to any changes, especially sudden changes in mood, behavior, thoughts, or feelings, or if you develop suicidal thoughts or actions. This is very important when an antidepressant medicine is started or when the dose is changed.
- Call your healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled. Call your healthcare provider between visits as needed, especially if you have concerns about symptoms.
Call your healthcare provider or get emergency medical help right away if you or your family member have any of the following symptoms, especially if they are new, worse, or worry you:
- attempts to commit suicide
- acting aggressive, being angry or violent
- new or worse depression
- panic attacks
- new or worse irritability
- an extreme increase in activity or talking (mania)
- acting on dangerous impulses
- thoughts about suicide or dying
- new or worse anxiety
- feeling very agitated or restless
- trouble sleeping
- other unusual changes in behavior or mood
What are mirtazapine tablets?
Mirtazapine tablets are prescription medicine used to treat a certain type of depression called Major Depressive Disorder
(MDD) in adults.
It is not known if mirtazapine tablets are safe and effective for use to treat MDD in children.
Who should not take mirtazapine tablets?
Do not take mirtazapine tablets if you:
- take a Monoamine Oxidase Inhibitor (MAOI)
- have stopped taking an MAOI in the last 14 days
- are being treated with the antibiotic linezolid or intravenous methylene blue
- if you are allergic to mirtazapine or any of the ingredients in mirtazapine tablets. See the end of this Medication Guide for a complete list of ingredients in mirtazapine tablets.
Ask your healthcare provider or pharmacist if you are not sure if you take an MAOI, including the antibiotic linezolid or intravenous methylene blue.
Do not start taking an MAOI for at least 14 days after you stop treatment with mirtazapine tablets.
Before taking mirtazapine tablets, tell your healthcare provider about all your medical conditions, including if you:
- have a history of suicide or depression
- have a history or family history of bipolar disorder, mania or hypomania
- have a low white blood cell count
- have glaucoma (high pressure in the eye)
- have or had heart problems or stroke
- have an abnormal heart beat called QT prolongation or a family
- history of QT prolongation
- have seizures
- have high cholesterol or triglyceride levels
- have low sodium levels in your blood
- have or had kidney or liver problems
- have low blood pressure
- are pregnant or plan to become pregnant. It is not known if mirtazapine tablets will harm your unborn baby.
- Talk to your healthcare provider if you become pregnant or think you may be pregnant during treatment with mirtazapine tablets.
- If you become pregnant while taking mirtazapine tablets, talk to your healthcare provider about registering with the National Pregnancy Registry for Antidepressants. You can register by calling 1-844-405-6185 or visiting online at https://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/antidepressants/. The purpose of this registry is to monitor the pregnancy outcomes in women who have been treated with mirtazapine tablets at any time during pregnancy.
- are breastfeeding or plan to breastfeed. Mirtazapine tablets may pass into your breast milk. Talk to your healthcare provider about the best way to feed your baby during treatment with mirtazapine tablets.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Mirtazapine tablets and other medicines may affect each other causing possible serious side effects.
Mirtazapine tablets may affect the way other medicines work and other medicines may affect the way mirtazapine tablets work.
Especially tell your healthcare provider if you take:
- MAOIs
- medicines to treat migraine headaches known as triptans
- tricyclic antidepressants
- fentanyl
- lithium
- tramadol
- tryptophan
- buspirone
- amphetamines
- benzodiazepines
- St. John’s Wort
- medicines used to treat mood, anxiety, psychotic or thought disorders, including selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs)
- medicines that may affect your heart rhythm (such as certain antibiotics and some antipsychotics)
Ask your healthcare provider if you are not sure if you are taking any of these medicines. Your healthcare provider can tell you if it is safe to take mirtazapine tablets with your other medicines.
Do not start or stop any other medicines during treatment with mirtazapine tablets without talking to your healthcare provider first. Stopping mirtazapine tablets suddenly may cause you to have serious side effects. See, “What are the possible side effects of mirtazapine tablets?”
Know the medicines you take. Keep a list of them to show to your healthcare provider and pharmacist when you get a new medicine.
How should I take mirtazapine tablets?
- Take mirtazapine tablets exactly as your healthcare provider tells you to. Do not change your dose or stop taking mirtazapine tablets without first talking to your healthcare provider.
- Your healthcare provider may need to change the dose of mirtazapine tablets until it is the right dose for you.
- Take mirtazapine tablets 1 time each day, preferably in the evening at bedtime.
- If you take too much mirtazapine tablets call your healthcare provider or poison control center at 1-800-222-1222 right away or go to the nearest hospital emergency room.
What should I avoid while taking mirtazapine tablets?
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how mirtazapine tablets affects you. Mirtazapine tablets can cause sleepiness or may affect your ability to make decisions, think clearly, or react quickly.
- Avoid drinking alcohol during treatment with mirtazapine tablets.
- Avoid taking medicines used to treat anxiety, insomnia, and seizures, called benzodiazepines, during treatment with mirtazapine tablets. Ask your healthcare provider if you are not sure if you take one of these medicines.
What are the possible side effects of mirtazapine tablets?
Mirtazapine tablets may cause serious side effects, including:
- See,“What is the most important information I should know about mirtazapine tablets?” ***Low white blood cell count.**Tell your healthcare provider right away if you develop any signs or symptoms of a low white blood cell count, including:
- fever
- chills
- sore throat
- mouth or nose sores
- flu-like symptoms
- infections ***Serotonin syndrome.A potentially life-threatening problem called serotonin syndrome can happen when you take mirtazapine tablets with certain other medicines. See,“Who should not take mirtazapine tablets?”**Stop taking mirtazapine tablets and call your healthcare provider or go to the nearest hospital emergency room right away if you have any of the following signs and symptoms of serotonin syndrome:
- agitation
- seeing or hearing things that are not real (hallucinations)
- confusion
- coma
- fast heart beat
- blood pressure changes
- dizziness
- sweating
- flushing
- high body temperature (hyperthermia)
- tremors, stiff muscles, or muscle twitching
- loss of coordination
- seizures
- nausea, vomiting, diarrhea ***Eye problems (angle-closure glaucoma).**Mirtazapine tablets may cause a certain type of eye problem called angle-closure glaucoma. Call your healthcare provider if you have eye pain, changes in your vision, or swelling or redness in or around the eye. Only some people are at risk for these problems. You may want to undergo an eye examination to see if you are at risk and receive preventative treatment if you are. *Heart rhythm problems. ***Severe skin reaction.**Mirtazapine tablets may cause a severe skin reaction that may include rash, fever, swollen glands, and other organ involvement such as liver, kidney, lung and heart. The reaction may sometimes be fatal. Tell your healthcare provider right away if you experience any of these signs. *Increased appetite and weight gain. *Sleepiness.See,“What should I avoid while taking mirtazapine tablets?” ***Mania or hypomania (manic episodes)**in people who have a history of bipolar disorder.
Symptoms may include:
- greatly increased energy
- severe trouble sleeping
- racing thoughts
- reckless behavior
- unusually grand ideas
- excessive happiness or irritability
- talking more or faster than usual *Seizures (convulsions). *Increased fat levels (cholesterol and triglycerides) in your blood. ***Low sodium levels in your blood (hyponatremia).**Low sodium levels in your blood may be serious and may cause death. Elderly people may be at greater risk for this. Signs and Symptoms of low sodium levels in your blood may include:
- headache
- difficulty concentrating
- memory changes
- confusion
- weakness and unsteadiness on your feet which can lead to falls *In severe or more sudden cases, signs and symptoms include:
- hallucinations (seeing or hearing things that are not real)
- fainting
- seizures
- coma
- respiratory arrest
- death *Changes in liver function tests. ***Discontinuation syndrome.**Suddenly stopping mirtazapine tablets may cause you to have serious side effects. Your healthcare provider may want to decrease your dose slowly. Symptoms may include:
- dizziness
- nausea and vomiting
- headache
- irritability and agitation
- problems sleeping
- abnormal dreams
- anxiety
- tiredness
- changes in your mood
- sweating
- confusion
- hypomania
- seizures
- electric shock sensation (paresthesia)
- ringing in your ears (tinnitus)
- shaking (tremor)
The most common side effects of mirtazapine tablets include:
- sleepiness
- increased appetite
- weight gain
- dizziness
These are not all the possible side effects of mirtazapine tablets.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store mirtazapine tablets?
- Store mirtazapine tablets at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep mirtazapine tablets away from light and moisture.
Keep mirtazapine tablets, and all medicines out of the reach of children.
General information about the safe and effective use of mirtazapine tablets.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use mirtazapine tablets for a condition for which it was not prescribed. Do not give mirtazapine tablets to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about mirtazapine tablets that is written for healthcare professionals.
What are the ingredients in mirtazapine tablets?
**Active ingredient:**mirtazapine, USP
**Inactive ingredients:**colloidal silicon dioxide, corn starch, hydroxypropyl cellulose, hypromellose 2910, lactose monohydrate, magnesium stearate, pregelatinized starch, and titanium dioxide. The 7.5 mg and 15 mg tablets also contains iron oxide (yellow) and the 30 mg tablets also contains iron oxides (yellow, red and black).
Manufactured for:
AvKARE
Pulaski, TN 38478
Mfg. Rev. 11/21
AV Rev. 09/25 (M)
DESCRIPTION SECTION
11 DESCRIPTION
Mirtazapine tablets contain mirtazapine, USP. Mirtazapine has a tetracyclic chemical structure and belongs to the piperazino-azepine group of compounds. It is designated 1,2,3,4,10,14b-hexahydro- 2-methylpyrazino [2,1-a] pyrido [2,3-c] [2] benzazepine and has the empirical formula of C 17H 19N 3. Its molecular weight is 265.35. The structural formula is the following and it is the racemic mixture:
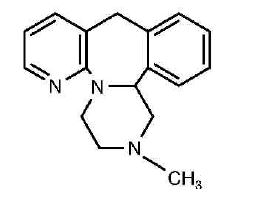
Mirtazapine, USP is a white to creamy white crystalline powder which is practically insoluble in water.
Mirtazapine tablets, USP are available for oral administration as scored film- coated tablets containing 15 mg or 30 mg of mirtazapine, USP, and unscored film-coated tablets containing 7.5 mg or 45 mg of mirtazapine, USP.
Each tablet contains colloidal silicon dioxide, corn starch, hydroxypropyl cellulose, hypromellose 2910, lactose monohydrate, magnesium stearate, pregelatinized starch, and titanium dioxide. The 7.5 mg and 15 mg tablets also contains iron oxide (yellow) and the 30 mg tablets also contains iron oxides (yellow, red and black).
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
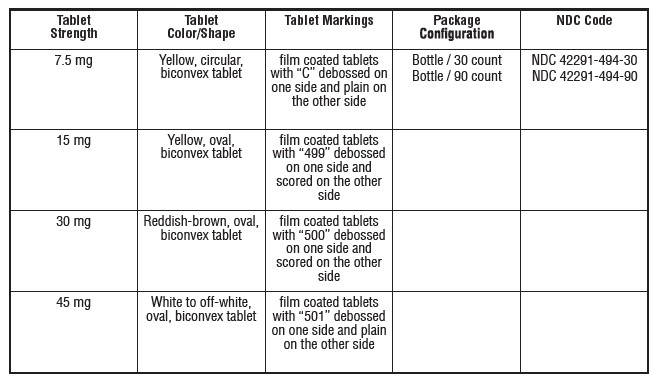
Mirtazapine tablets, USP are supplied as:
Storage
Store at 20° to 25°C (68° to 77°F); excursions permitted 15° to 30°C (59° to 86°F). [see USP Controlled Room Temperature]. Protect from light and moisture.
