CERIANNA
These highlights do not include all the information needed to use CERIANNA™ safely and effectively. See full prescribing information for CERIANNA. CERIANNA™ (fluoroestradiol F 18) Injection, for intravenous use Initial U.S. Approval: 2020
b2c2a2df-cf3e-4a29-a033-231ed5a94b58
HUMAN PRESCRIPTION DRUG LABEL
Sep 10, 2025
GE Healthcare Inc.
DUNS: 053046579
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Fluoroestradiol F 18
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 50 mL Vial Shield Label
NDC 72874-001-01
Multiple-Dose Vial
CERIANNA™ (fluoroestradiol F 18) Injection
148 MBq/mL to 3,700 MBq/mL (4 mCi/mL to 100 mCi/mL) @ EOS*
For Intravenous Use Only
Sterile, Non-pyrogenic
Diagnostic
Date/time of calibration: _____________; ________hr:min
Expiration date & time: _____________; ________hr:min
Volume: _______________________ mL
Lot # ________________________
Concentration: ___________mCi/mL
at time of calibration
Total Activity: ____________mCi at
time of calibration
Contains: 148 MBq/mL to 3,700 MBq/mL (4 mCi/mL to 100
mCi/mL) of no-carrier added fluoroestradiol F 18 @ EOS*;
sodium ascorbate 0.44% w/v in sodium chloride 0.9% w/v
and ethanol no more than 3.2% w/v
Usual dosage: See prescribing information
Do not use if cloudy or if it contains particulate matter
*EOS = End of Synthesis
CAUTION: RADIOACTIVE MATERIAL
Expires 12 hours after EOS*
Store at 20°C to 25°C (68°F to 77°F)
Store upright in a shielded container
Aseptically withdraw and handle doses
[18F] Half-Life = 109.8 minutes
Calculate correct dosage from date
and time of calibration
Dist. by: GE Healthcare, Inc.
Arlington Heights, IL 60004 USA
GE is a trademark of General Electric
Company used under trademark license.
100113-0B
Rx ONLY

INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
CERIANNA is indicated for use with positron emission tomography (PET) imaging for the detection of estrogen receptor (ER)-positive lesions as an adjunct to biopsy in patients with recurrent or metastatic breast cancer.
Limitations of Use
Tissue biopsy should be used to confirm recurrence of breast cancer and to verify ER status by pathology. CERIANNA is not useful for imaging other receptors, such as human epidermal growth factor receptor 2 (HER2) and the progesterone receptor (PR).
CERIANNA is a radioactive diagnostic agent indicated for use with positron emission tomography (PET) imaging for the detection of estrogen receptor (ER)-positive lesions as an adjunct to biopsy in patients with recurrent or metastatic breast cancer. (1)
Limitations of Use
Tissue biopsy should be used to confirm recurrence of breast cancer and to verify ER status by pathology. CERIANNA is not useful for imaging other receptors, such as human epidermal growth factor receptor 2 (HER2) and the progesterone receptor (PR). (1, 5.1)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
None.
- None. (4)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Misdiagnosis
Inadequate Tumor Characterization and Other ER-Positive Pathology
Breast cancer may be heterogeneous within patients and across time. CERIANNA images ER and is not useful for imaging other receptors such as HER2 and PR. The uptake of fluoroestradiol F 18 is not specific for breast cancer and may occur in a variety of ER-positive tumors that arise outside of the breast, including from the uterus and ovaries. Do not use CERIANNA in lieu of biopsy when biopsy is indicated in patients with recurrent or metastatic breast cancer.
False Negative CERIANNA Scan
A negative CERIANNA scan does not rule out ER-positive breast cancer [see Clinical Studies (14)]. Pathology or clinical characteristics that suggest a patient may benefit from systemic hormone therapy should take precedence over a discordant negative CERIANNA scan.
5.2 Radiation Risks
Diagnostic radiopharmaceuticals, including CERIANNA, expose patients to radiation [see Dosage and Administration (2.6)]. Radiation exposure is associated with a dose-dependent increased risk of cancer. Ensure safe drug handling and patient preparation procedures to protect patients and health care providers from unintentional radiation exposure [see Dosage and Administration (2.1) and (2.3)].
- Risk of Misdiagnosis. Do not use CERIANNA in lieu of biopsy when biopsy is indicated in patients with recurrent or metastatic breast cancer. Pathology or clinical characteristics that suggest a patient may benefit from systemic hormone therapy should take precedence over a discordant negative CERIANNA scan. (5.1)
- Radiation Risks. Ensure safe drug handling and patient preparation procedures to protect patients and health care providers from unintentional radiation exposure. (2.1, 2.3, 5.2)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of CERIANNA was evaluated from published clinical studies of 1,207 patients with breast cancer receiving at least one fluoroestradiol F 18 administration. The following adverse reactions occurred at a rate < 1%:
- General disorders: injection-site pain
- Neurological and gastrointestinal disorders: dysgeusia
Reported adverse reactions include: injection-site pain and dysgeusia (6)
To report SUSPECTED ADVERSE REACTIONS, contact GE HealthCare at 1-800-654-0118 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
Drugs that bind to the estrogen receptor (ER) may compete with the binding of fluoroestradiol F 18 and may reduce the detection of ER-positive lesions with CERIANNA.
Before administering CERIANNA, discontinue drugs that bind to the ER, such as SERMs and SERDs, for at least 5 biological half-lives (e.g., elacestrant for 11 days, tamoxifen for 8 weeks, and fulvestrant for 28 weeks) [see Dosage and Administration (2.3)].
- Before administering CERIANNA, discontinue drugs that bind to the ER, such as SERMs and SERDs, for at least 5 biological half-lives (e.g., elacestrant for 11 days, tamoxifen for 8 weeks, and fulvestrant for 28 weeks). (2.3, 7)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
All radiopharmaceuticals, including CERIANNA, have the potential to cause fetal harm depending on the fetal stage of development and the magnitude of radiation dose. Advise a pregnant woman of the potential risks of fetal exposure to radiation from administration of CERIANNA.
There are no available data on CERIANNA use in pregnant women. No animal reproduction studies using fluoroestradiol F 18 have been conducted to evaluate its effect on female reproduction and embryo-fetal development.
The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defects, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of fluoroestradiol F 18 in human milk, or its effects on the breastfed infant or milk production. Lactation studies have not been conducted in animals. Advise a lactating woman to avoid breastfeeding for 4 hours after CERIANNA administration in order to minimize radiation exposure to a breastfed infant.
8.4 Pediatric Use
The safety and effectiveness of CERIANNA in pediatric patients have not been established.
8.5 Geriatric Use
Clinical studies of fluoroestradiol F 18 injection did not reveal any difference in pharmacokinetics or biodistribution in patients aged 65 and over.
- Lactation: Interrupt breastfeeding. Advise a lactating woman to avoid breastfeeding for 4 hours after CERIANNA administration. (8.2)
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
CERIANNA is supplied in a 50 mL multiple-dose glass vial (NDC 72874-001-01) containing a clear, colorless injection solution at a strength of 148 MBq/mL to 3,700 MBq/mL (4 mCi/mL to 100 mCi/mL) fluoroestradiol F 18 at the end of synthesis. Each vial contains multiple doses and is enclosed in a shield container to minimize external radiation exposure.
16.2 Storage and Handling
Storage
Store CERIANNA at controlled room temperature (USP) 20°C to 25°C (68°F to 77°F). Store CERIANNA upright in the original container with radiation shielding. The expiration date and time are provided on the container label. Use CERIANNA within 12 hours from the time of the end of synthesis.
Handling
This preparation is approved for use by persons under license by the Nuclear Regulatory Commission or the relevant regulatory authority of an Agreement State.
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Radiation Safety - Drug Handling
CERIANNA is a radioactive drug. Only authorized persons qualified by training and experience should receive, use, and administer CERIANNA. Handle CERIANNA with appropriate safety measures to minimize radiation exposure during administration [see Warnings and Precautions (5.2)]. Use waterproof gloves and effective radiation shielding, including syringe shields, when preparing and handling CERIANNA.
2.2 Recommended Dosage and Administration Instructions
Recommended Dosage
The recommended amount of radioactivity to be administered for PET imaging is 222 MBq (6 mCi), with a range of 111 MBq to 222 MBq (3 mCi to 6 mCi), administered as a single intravenous injection of 10 mL or less over 1 to 2 minutes.
Preparation and Administration
- For patient preparation instructions, see Dosage and Administration 2.3.
- Use aseptic technique and radiation shielding when withdrawing and administering CERIANNA.
- Visually inspect the radiopharmaceutical solution. Do not use if it contains particulate matter or if it is cloudy or discolored (CERIANNA is a clear, colorless solution).
- CERIANNA may be diluted with 0.9% Sodium Chloride Injection, USP.
- Assay the dose in a suitable dose calibrator prior to administration.
Post-Administration Instructions
- Follow the CERIANNA injection with an intravenous flush of 0.9% Sodium Chloride injection, USP.
- Dispose of any unused CERIANNA in compliance with applicable regulations.
2.3 Patient Preparation
Assessment for Drug Interactions
Before administering CERIANNA, discontinue drugs that bind to ER (e.g., selective estrogen receptor modulators (SERMs) and selective estrogen receptor down-regulators (SERDs)) [see Drug Interactions (7)].
Patient Hydration and Voiding
Instruct patients to drink water to ensure adequate hydration prior to administration of CERIANNA and to continue drinking and voiding frequently during the first hours following administration to reduce radiation exposure.
Pregnancy Status
Assessment of pregnancy status is recommended in females of reproductive potential before administering CERIANNA.
2.4 Image Acquisition
Position the patient supine with arms above the head, if possible. The recommended start time for image acquisition is 80 minutes after the intravenous administration of CERIANNA. Scan duration adapted from the range of 20 minutes to 30 minutes and imaging start times adapted within the range of 20 minutes to 80 minutes may be customized according to the equipment used and patient and tumor characteristics for optimal image quality.
2.5 Image Interpretation
Uptake of fluoroestradiol F 18 depends on ER density and function in tumors and physiologic tissue, including in liver, ovary, and uterus. Detection of ER-positive tumors should be based on comparison with tissue background outside of organs with high physiologic uptake and regions with high activity due to hepatobiliary and urinary excretion.
2.6 Radiation Dosimetry
Radiation absorbed dose estimates are shown in Table 1 for organs and tissues of adults from intravenous administration of CERIANNA. The radiation effective dose resulting from administration of 222 MBq (6 mCi) of CERIANNA to an adult weighing 70 kg is estimated to be 4.9 mSv. Critical organs include the liver, gallbladder, and uterus. When PET/CT is performed, exposure to radiation will increase by an amount dependent on the settings used for the CT acquisition.
Table 1. Estimated Radiation Absorbed Doses in Various Organs/Tissues in Adults Who Received FLUOROESTRADIOL F 18|
Organ |
Mean Absorbed Dose Per Unit of Activity Administered (mGy/MBq) |
|---|---|
|
Adrenals |
0.023 |
|
Brain |
0.01 |
|
Breasts |
0.009 |
|
Gallbladder |
0.102 |
|
Lower large intestine |
0.012 |
|
Small intestine |
0.027 |
|
Stomach |
0.014 |
|
Upper large intestine |
0.03 |
|
Heart wall |
0.026 |
|
Kidney |
0.035 |
|
Liver |
0.126 |
|
Lungs |
0.017 |
|
Muscle |
0.021 |
|
Ovaries |
0.018 |
|
Pancreas |
0.023 |
|
Red Marrow |
0.013 |
|
Bone surface |
0.014 |
|
Skin |
0.005 |
|
Spleen |
0.015 |
|
Testes |
0.012 |
|
Thymus |
0.014 |
|
Thyroid |
0.012 |
|
Urinary bladder |
0.05 |
|
Uterus |
0.039 |
|
Lens |
0.009 |
|
Effective dose = 0.022 mSv/MBq |
- Recommended dose is 222 MBq (6 mCi), with a range of 111 MBq to 222 MBq (3 mCi to 6 mCi), administered as an intravenous injection over 1 to 2 minutes. (2.2)
- Recommended imaging start time is 80 minutes (range 20 minutes to 80 minutes) after drug administration. (2.4)
- See full prescribing information for additional preparation, administration, imaging, and radiation dosimetry information. (2)
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
Injection: clear, colorless solution in a multiple-dose vial containing 148 MBq/mL to 3,700 MBq/mL (4 mCi/mL to 100 mCi/mL) of fluoroestradiol F 18 at end of synthesis.
Injection: 148 MBq/mL to 3,700 MBq/mL (4 mCi/mL to 100 mCi/mL) of fluoroestradiol F 18 in a multiple-dose vial. (3)
DESCRIPTION SECTION
11 DESCRIPTION
11.1 Chemical Characteristics
CERIANNA contains fluoroestradiol fluorine 18 (F 18), a synthetic estrogen analog. Chemically, fluoroestradiol F 18 is [18F]16α-fluoro-3,17β-diol- estratriene-1,3,5(10). The molecular weight is 289.37, and the structural formula is:
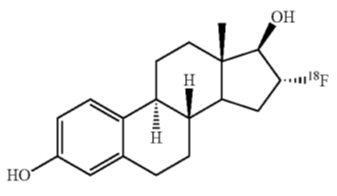
CERIANNA is a sterile, clear, colorless solution for intravenous injection, with an osmolarity of 340 mOsm. Its pH ranges between 5.5 to 8.0. The composition of the final product in 40 mL solution is fluoroestradiol no more than 5 mcg, fluoroestradiol F 18 148 MBq/mL to 3,700 MBq/mL (4 mCi/mL to 100 mCi/mL), sodium ascorbate 0.44% w/v in sodium chloride 0.9% w/v, and ethanol no more than 3.2% w/v.
11.2 Physical Characteristics
CERIANNA is radiolabeled with F 18, a cyclotron produced radionuclide that decays by positron emission to stable oxygen 18 with a half-life of 109.8 minutes. The principal photons useful for diagnostic imaging are the coincident pair of 511 keV gamma photons, resulting from the interaction of the emitted positron with an electron (Table 2).
Table 2. Principal Radiation Produced From Decay of Fluorine 18 Radiation|
Radiation |
Energy Level (keV) |
% Abundance |
|---|---|---|
|
Positron |
249.8 |
96.9 |
|
Gamma |
511 |
193.5 |
11.3 External Radiation
The point source air-kerma coefficient for F 18 is 3.75 × 10-17 Gy m2 / (Bq s). The first half-value thickness of lead (Pb) for F 18 gamma rays is approximately 6 mm. The relative reduction of radiation emitted by F 18 that results from various thicknesses of lead shielding is shown in Table 3. The use of 8 cm Pb decreases the radiation transmission (i.e., exposure) by a factor of about 10,000.
Table 3. Radiation Attenuation of 511 keV Gamma Rays by Lead Shielding|
Shield Thickness cm of Lead (Pb) |
Coefficient of Attenuation |
|---|---|
|
0.6 |
0.5 |
|
2 |
0.1 |
|
4 |
0.01 |
|
6 |
0.001 |
|
8 |
0.0001 |
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
The effectiveness of CERIANNA for detecting ER-positive non-primary breast cancer lesions was evaluated based on published study reports of fluoroestradiol F 18. Study 1 (NCT01986569) enrolled 90 women (median age 55 years, 39% premenopausal) with histologically confirmed invasive breast cancer. The patients had first known or suspected recurrence of treated breast cancer or stage IV metastatic breast cancer. Recent biopsy of lesions outside of bone and areas with high physiologic fluoroestradiol F 18 uptake was also required [see Dosage and Administration (2.5)]. Patients concurrently using estrogen receptor modulators or fulvestrant discontinued them 60 days prior to fluoroestradiol F 18 administration. Concurrent use of aromatase inhibitors was permitted. Three image readers were blinded to all clinical information, except for the location of the largest biopsied lesion, for which pathologists independently provided an Allred score (0 to 8). The image readers scored the intensity of FES uptake on a three-point scale relative to normal biodistribution as either "decreased," "equivocal," or "increased" (1 to 3).
Image reader performance for distinguishing between ER-positive and ER- negative fluoroestradiol F 18 uptake was compared to biopsy in 85 patients. Of the 47 patients with positive biopsy (Allred score ≥ 3), 36 were positive on imaging (majority reader score = 3). Ten of 11 patients with false negative imaging had Allred scores between 3 and 6 [see Warnings and Precautions (5.1)]. Of the 38 patients with negative biopsy, all 38 were negative on imaging.
Study 2 (NCT00602043) in 13 patients showed similar results.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Radiation Risks
Advise patients of the radiation risks of CERIANNA [see Warnings and Precautions (5.2)]. Instruct patients to drink water to ensure adequate hydration prior to administration of CERIANNA and to continue drinking and voiding frequently during the first hours following administration to reduce radiation exposure [see Dosage and Administration (2.3)].
Pregnancy
Advise a pregnant woman of the potential risks of fetal exposure to radiation doses with CERIANNA [see Use in Specific Populations (8.1)].
Lactation
Advise a lactating woman to avoid breastfeeding for 4 hours after CERIANNA administration in order to minimize radiation exposure to a breastfed infant [see Use in Specific Populations (8.2)].
SPL UNCLASSIFIED SECTION
© 2025 GE HealthCare
Distributed by GE Healthcare Inc., Arlington Heights, IL 60004 U.S.A.
GE is a trademark of General Electric Company used under trademark license.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Fluoroestradiol F 18 binds ER. The following binding affinity: Kd = 0.13 ± 0.02 nM, Bmax = 1901 ± 89 fmol/mg, and IC50 = 0.085 nM, was determined in an ER-positive human breast cancer cell line (MCF-7).
12.2 Pharmacodynamics
The relationship between fluoroestradiol F18 plasma concentrations and image interpretation has not been studied. Fluoroestradiol F18 uptake measured by PET in human tumors is directly proportional to tumor ER expression measured by in vitro assays.
12.3 Pharmacokinetics
Distribution
After intravenous injection, 95% of fluoroestradiol F 18 is bound to plasma proteins. Fluoroestradiol F 18 distributes primarily to hepatobiliary system, and also to small and large intestines, heart wall, blood, kidney, uterus and bladder.
Metabolism
Fluoroestradiol F 18 is metabolized in the liver. At 20 minutes after injection, approximately 20% of circulating radioactivity in the plasma is in the form of non-metabolized fluoroestradiol F 18. At 2 hours after injection, circulating fluoroestradiol F 18 levels are less than 5% of peak concentration.
Excretion
Elimination is by biliary and urinary excretion.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
No long-term studies in animals were performed to evaluate the carcinogenic potential of CERIANNA.
Mutagenesis
Fluoroestradiol was evaluated by in vitro bacterial reverse mutation assay (Ames test) and in vitro L5178Y/TK+/- mouse lymphoma mutagenesis assay. Fluoroestradiol was negative for genotoxicity by Ames test at up to 1.25 µg per plate for 5 tester strains (Salmonella typhimurium tester strains TA98, TA100, TA1535 and TA1537 and Escherichia Coli tester strain WP2 uvrA) in the presence or absence of S9 metabolic activation. Fluoroestradiol was negative for genotoxicity by L5178Y/TK+/- mouse lymphoma mutagenesis assay at up to 8 ng/mL in the absence or presence of S9 metabolic activation.
Potential in vivo genotoxicity of fluoroestradiol was evaluated in a rat micronucleus assay. In this assay, fluoroestradiol did not increase the number of micronucleated polychromatic erythrocytes (MN-PCEs) at 51 µg/kg/day, when given for 14 consecutive days. However, CERIANNA has the potential to be mutagenic because of the F 18 radioisotope.
Impairment of Fertility
No studies in animals have been performed to evaluate potential impairment of fertility in males or females.
