Lisinopril
These highlights do not include all the information needed to use LISINOPRIL TABLETS safely and effectively. See full prescribing information for LISINOPRIL TABLETS. LISINOPRIL tablets, for oral use Initial U.S. Approval: 1988
34953f77-bae1-41fe-af11-3ea0b3ceaa11
HUMAN PRESCRIPTION DRUG LABEL
Aug 13, 2025
A-S Medication Solutions
DUNS: 830016429
Products 5
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Lisinopril
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Lisinopril
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Lisinopril
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Lisinopril
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Lisinopril
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Lisinopril

STORAGE AND HANDLING SECTION
Storage
Store at controlled room temperature, 20° to 25°C (68° to 77°F) [see USP]. Protect from moisture, freezing and excessive heat. Dispense in a tight container.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Product: 50090-0808
NDC: 50090-0808-0 30 TABLET in a BOTTLE
NDC: 50090-0808-2 90 TABLET in a BOTTLE
NDC: 50090-0808-3 100 TABLET in a BOTTLE
NDC: 50090-0808-1 100 TABLET in a BOTTLE
Product: 50090-2228
Product: 50090-2988
NDC: 50090-2988-0 30 TABLET in a BOTTLE
NDC: 50090-2988-3 60 TABLET in a BOTTLE
NDC: 50090-2988-4 200 TABLET in a BOTTLE
NDC: 50090-2988-5 90 TABLET in a BOTTLE
NDC: 50090-2988-1 100 TABLET in a BOTTLE
Product: 50090-2989
Product: 50090-2990
DESCRIPTION SECTION
11 DESCRIPTION
Lisinopril is an oral long-acting angiotensin converting enzyme (ACE) inhibitor. Lisinopril, a synthetic peptide derivative, is chemically described as (S)-1-[N2-(1-carboxy-3-phenylpropyl)-L-lysyl]-L-proline dihydrate. Its empirical formula is C21H31N3O52H2O and its structural formula is:
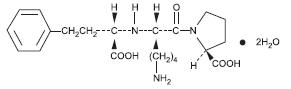
Lisinopril is a white, crystalline powder, with a molecular weight of 441.53. It is soluble in water and sparingly soluble in methanol and practically insoluble in ethanol.
Lisinopril tablets USP are supplied as 2.5 mg, 5 mg, 10 mg, 20 mg, 30 mg and 40 mg tablets for oral administration.
Inactive Ingredients:
2.5 mg tablets - colloidal silicon dioxide, dibasic calcium phosphate, magnesium stearate, mannitol, pre-gelatinized starch and starch (corn).
5 mg, 10 mg, 20 mg and 30 mg tablets – colloidal silicon dioxide, dibasic calcium phosphate, magnesium stearate, mannitol, red ferric oxide, pre- gelatinized starch and starch (corn).
40 mg tablets - colloidal silicon dioxide, dibasic calcium phosphate, magnesium stearate, mannitol, yellow ferric oxide, pre-gelatinized starch and starch (corn).
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
NOTE: This information is intended to aid in the safe and effective use of this medication. It is not a disclosure of all possible adverse or intended effects.
Pregnancy
Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to notify their healthcare provider with a known or suspected pregnancy [see WARNINGS AND PRECAUTIONS (5.1) and USE IN SPECIFIC POPULATIONS (8.1)].
Angioedema
Angioedema, including laryngeal edema may occur at any time during treatment with angiotensin converting enzyme inhibitors, including lisinopril. Tell patients to report immediately any signs or symptoms suggesting angioedema (swelling of face, extremities, eyes, lips, tongue, difficulty in swallowing or breathing) and to take no more drug until they have consulted with the prescribing physician.
Lactation
Advise women not to breastfeed during treatment with lisinopril [see USE IN SPECIFIC POPULATIONS (8.2)].
Symptomatic Hypotension
Tell patients to report light-headedness especially during the first few days of therapy. If actual syncope occurs, tell the patient to discontinue the drug until they have consulted with the prescribing physician.
Tell patients that excessive perspiration and dehydration may lead to an excessive fall in blood pressure because of reduction in fluid volume. Other causes of volume depletion such as vomiting or diarrhea may also lead to a fall in blood pressure; advise patients accordingly.
Hyperkalemia
Tell patients not to use salt substitutes containing potassium without consulting their physician.
Hypoglycemia
Tell diabetic patients treated with oral antidiabetic agents or insulin starting an ACE inhibitor to monitor for hypoglycaemia closely, especially during the first month of combined use [see DRUG INTERACTIONS (7.2)].
Leukopenia/Neutropenia
Tell patients to report promptly any indication of infection (e.g., sore throat, fever), which may be a sign of leukopenia/neutropenia.
LUPIN and the
 are registered trademarks of Lupin Pharmaceuticals, Inc.
are registered trademarks of Lupin Pharmaceuticals, Inc.
Manufactured for:
Lupin Pharmaceuticals, Inc.
Naples, FL 34108
United States.
MADE IN INDIA
Revised: June 2024 ID#: 276103
