Famotidine
Famotidine Tablets USP
85c7d20d-3dec-b918-4c1d-8ca1db214e48
HUMAN PRESCRIPTION DRUG LABEL
Jan 9, 2024
AvPAK
DUNS: 832926666
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Famotidine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
Famotidine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
Drug Labeling Information
HOW SUPPLIED SECTION
HOW SUPPLIED
Famotidine Tablets USP (white round tablets) containing 20mg of famotidine and
engraved with .
.
NDC 50268-303-15 10 Tablets per card, 5 cards per carton
Famotidine Tablets USP (white round tablets) containing 40mg of famotidine and
engraved with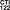 .
.
NDC 50268-304-15 10 Tablets per card, 5 cards per carton
Dispensed in Blister Punch Material. For Institutional Use Only.
STORAGE
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Manufactured for:
AvKARE, Inc.
Pulaski, TN 38478
Mfg. Rev. 06/12 AV 09/14 (P)
CTI-12 Rev. C
