Moxifloxacin
These highlights do not include all the information needed to use MOXIFLOXACIN OPHTHALMIC SOLUTION, USP safely and effectively. See full prescribing information for MOXIFLOXACIN OPHTHALMIC SOLUTION, USP.MOXIFLOXACIN ophthalmic solution, USP 0.5% Sterile topical ophthalmic solutionInitial U.S. Approval: 1999
8f7a0c4c-34f4-7362-579b-f26c3338f792
HUMAN PRESCRIPTION DRUG LABEL
Sep 19, 2025
Apotex Corp.
DUNS: 845263701
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
moxifloxacin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL- BOTTLE LABEL
Apotex Corp.NDC 60505-0582-4
Moxifloxacin Ophthalmic Solution, USP
****0.5%
3 mL
STERILE

WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
In patients receiving systemically administered quinolones, including moxifloxacin, serious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported, some following the first dose. Some reactions were accompanied by cardiovascular collapse, loss of consciousness, angioedema (including laryngeal, pharyngeal or facial edema), airway obstruction, dyspnea, urticaria, and itching. If an allergic reaction to moxifloxacin occurs, discontinue use of the drug. Serious acute hypersensitivity reactions may require immediate emergency treatment. Oxygen and airway management should be administered as clinically indicated.
5.2 Growth of Resistant Organisms With Prolonged Use
As with other anti-infectives, prolonged use may result in overgrowth of non- susceptible organisms, including fungi. If superinfection occurs, discontinue use and institute alternative therapy. Whenever clinical judgment dictates, the patient should be examined with the aid of magnification, such as slit- lamp biomicroscopy, and, where appropriate, fluorescein staining.
5.3 Avoidance of Contact Lens Wear
Patients should be advised not to wear contact lenses if they have signs or symptoms of bacterial conjunctivitis.
- Hypersensitivity Reactions: Hypersensitivity and anaphylaxis have been reported with systemic use of moxifloxacin. (5.1)
- Prolonged Use: May result in overgrowth of non-susceptible organisms, including fungi. If superinfection occurs, discontinue use and institute alternative therapy. (5.2)
- Avoid Contact Lens Wear: Patients should not wear contact lenses if they have signs or symptoms of bacterial conjunctivitis. (5.3)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies with moxifloxacin ophthalmic solution in pregnant women to inform any drug-associated risks.
Oral administration of moxifloxacin to pregnant rats and monkeys and intravenously to pregnant rabbits during the period of organogenesis did not produce adverse maternal or fetal effects at clinically relevant doses. Oral administration of moxifloxacin to pregnant rats during late gestation through lactation did not produce adverse maternal, fetal or neonatal effects at clinically relevant doses (see Data).
Data
Animal Data
Embryo-fetal studies were conducted in pregnant rats administered with 20, 100, or 500 mg/kg/day moxifloxacin by oral gavage on Gestation Days 6 to 17, to target the period of organogenesis. Decreased fetal body weight and delayed skeletal development were observed at 500 mg/kg/day [277 times the human area under the curve (AUC) at the recommended human ophthalmic dose]. The No- Observed-Adverse-Effect-Level (NOAEL) for developmental toxicity was 100 mg/kg/day (30 times the human AUC at the recommended human ophthalmic dose).
Embryo-fetal studies were conducted in pregnant rabbits administered with 2, 6.5, or 20 mg/kg/day moxifloxacin by intravenous administration on Gestation Days 6 to 20, to target the period of organogenesis. Abortions, increased incidence of fetal malformations, delayed fetal skeletal ossification, and reduced placental and fetal body weights were observed at 20 mg/kg/day (1086 times the human AUC at the recommended human ophthalmic dose), a dose that produced maternal body weight loss and death. The NOAEL for developmental toxicity was 6.5 mg/kg/day (246 times the human AUC at the recommended human ophthalmic dose).
Pregnant cynomolgus monkeys were administered moxifloxacin at doses of 10, 30, or 100 mg/kg/day by intragastric intubation between Gestation Days 20 and 50, targeting the period of organogenesis. At the maternal toxic doses of ≥ 30 mg/kg/day, increased abortion, vomiting, and diarrhea were observed. Smaller fetuses/reduced fetal body weights were observed at 100 mg/kg/day (2864 times the human AUC at the recommended human ophthalmic dose). The NOAEL for fetal toxicity was 10 mg/kg/day (174 times the human AUC at the recommended human ophthalmic dose).
In a pre- and postnatal study, rats were administered moxifloxacin by oral gavage at doses of 20, 100, and 500 mg/kg/day from Gestation Day 6 until the end of lactation. Maternal death occurred during gestation at 500 mg/kg/day. Slight increases in the duration of pregnancy, reduced pup birth weight, and decreased prenatal and neonatal survival were observed at 500 mg/kg/day (estimated 277 times the human AUC at the recommended human ophthalmic dose). The NOAEL for pre- and postnatal development was 100 mg/kg/day (estimated 30 times the human AUC at the recommended human ophthalmic dose).
8.2 Lactation
Risk Summary
There is no data regarding the presence of moxifloxacin ophthalmic solution in human milk, the effects on the breastfed infants, or the effects on milk production/excretion to inform risk of moxifloxacin ophthalmic solution to an infant during lactation.
A study in lactating rats has shown transfer of moxifloxacin into milk following oral administration.
Systemic levels of moxifloxacin following topical ocular administration are low [see Clinical Pharmacology (12.3)], and it is not known whether measurable levels of moxifloxacin would be present in maternal milk following topical ocular administration.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for moxifloxacin ophthalmic solution and any potential adverse effects on the breastfed child from moxifloxacin ophthalmic solution.
8.4 Pediatric Use
The safety and effectiveness of moxifloxacin ophthalmic solution 0.5% have been established in all ages. Use of moxifloxacin ophthalmic solution is supported by evidence from adequate and well controlled studies of moxifloxacin ophthalmic solution in adults, children, and neonates [see Clinical Studies (14)].
There is no evidence that the ophthalmic administration of moxifloxacin ophthalmic solution has any effect on weight bearing joints, even though oral administration of some quinolones has been shown to cause arthropathy in immature animals.
8.5 Geriatric Use
No overall differences in safety and effectiveness have been observed between elderly and younger patients.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term studies in animals to determine the carcinogenic potential of moxifloxacin have not been performed. However, in an accelerated study with initiators and promoters, moxifloxacin was not carcinogenic in rats following up to 38 weeks of oral dosing at 500 mg/kg/day (3224 times the highest recommended total daily human ophthalmic dose for a 60 kg person, based on body surface area).
Mutagenesis
Moxifloxacin was not mutagenic in four bacterial strains used in the Ames Salmonella reversion assay. As with other quinolones, the positive response observed with moxifloxacin in strain TA 102 using the same assay may be due to the inhibition of DNA gyrase. Moxifloxacin was not mutagenic in the CHO/HGPRT mammalian cell gene mutation assay. An equivocal result was obtained in the same assay when V79 cells were used. Moxifloxacin was clastogenic in the V79 chromosome aberration assay, but it did not induce unscheduled DNA synthesis in cultured rat hepatocytes. There was no evidence of genotoxicity in vivo in a micronucleus test or a dominant lethal test in mice.
Impairment of Fertility
Moxifloxacin had no effect on fertility in male and female rats at oral doses as high as 500 mg/kg/day, approximately 3224 times the highest recommended total daily human ophthalmic dose, based on body surface area. At 500 mg/kg/day orally, there were slight effects on sperm morphology (head-tail separation) in male rats and on the estrous cycle in female rats.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Avoid Contamination of the Product
Advise patients not to touch the dropper tip to any surface to avoid contaminating the contents.
Avoid Contact Lens Wear
Advise patients not to wear contact lenses if they have signs and symptoms of bacterial conjunctivitis [see Warnings and Precautions (5.3)].
Hypersensitivity Reactions
Systemically administered quinolones including moxifloxacin have been associated with hypersensitivity reactions, even following a single dose. Instruct patients to discontinue use immediately and contact their physician at the first sign of a rash or allergic reaction [see Warnings and Precautions (5.1)].
Rx Only
|
Manufactured by: |
Manufactured for: |
|
Apotex Inc. |
Apotex Corp. |
|
Toronto, Ontario |
Weston, Florida |
|
Canada |
33326 |
|
M9L 1T9 |
Revised: April 2023
DESCRIPTION SECTION
11 DESCRIPTION
Moxifloxacin ophthalmic solution, USP 0.5% is a sterile solution for topical ophthalmic use. Moxifloxacin hydrochloride is an 8-methoxy fluoroquinolone anti-infective, with a diazabicyclononyl ring at the C7 position.
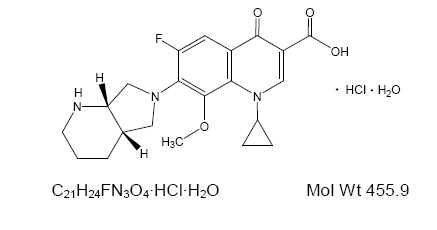
Chemical Name: 1-Cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-[(4aS,7aS)-octahydro-6H-pyrrolol[3,4-b]pyridin-6-yl]-4-oxo-3-quinoline carboxylic acid, monohydrochloride monohydrate.
Moxifloxacin hydrochloride monohydrate is a light yellow or yellow powder or crystals. Each mL of moxifloxacin ophthalmic solution, USP contains 5.45 mg moxifloxacin hydrochloride, equivalent to 5 mg moxifloxacin base.
**Contains: Active:**Moxifloxacin 0.5% (5 mg/mL);**Inactives:**Boric acid, sodium chloride, sodium hydroxide and water for injection. May also contain hydrochloric acid and/or additional sodium hydroxide to adjust pH to approximately 6.8.
Moxifloxacin ophthalmic solution, USP is an isotonic solution with an osmolality of approximately 290 mOsm/kg.
