Mesalamine
MESALAMINE SUPPOSITORIES
Approved
Approval ID
aa63b4bb-2de7-40aa-b26b-1b00b0183eac
Product Type
HUMAN PRESCRIPTION DRUG LABEL
Effective Date
Oct 31, 2022
Manufacturers
FDA
Zydus Lifesciences Limited
DUNS: 918596198
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Mesalamine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
NDC Product Code70771-1513
Application NumberANDA208953
Product Classification
M
Marketing Category
C73584
G
Generic Name
Mesalamine
Product Specifications
Route of AdministrationRECTAL
Effective DateOctober 31, 2022
FDA Product Classification
INGREDIENTS (2)
FAT, HARDInactive
Code: 8334LX7S21
Classification: IACT
MESALAMINEActive
Quantity: 1000 mg in 1 1
Code: 4Q81I59GXC
Classification: ACTIB
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
LOINC: 51945-4Updated: 2/13/2020
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
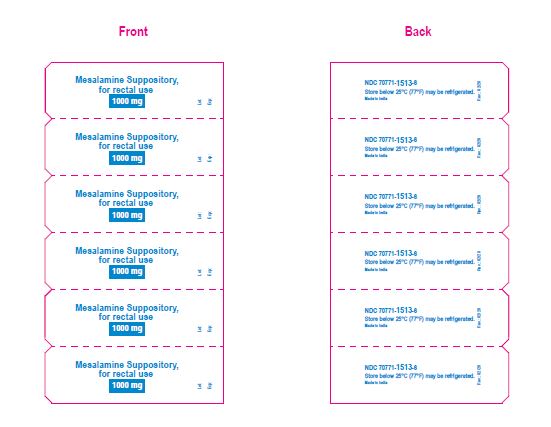
NDC 70771-1513-6
Mesalamine suppositories, 1000 mg
30 rectal suppositories
Rx only
Image
NDC 70771-1513-7
Mesalamine rectal suppositories, 1000 mg
30 rectal suppositories
Rx only

Image
SPL UNCLASSIFIED SECTION
LOINC: 42229-5Updated: 9/4/2020
