Pregabalin
These highlights do not include all the information needed to use PREGABALIN CAPSULES safely and effectively. See full prescribing information for PREGABALIN CAPSULES. PREGABALIN capsules, for oral use CV Initial U.S. Approval: 2004
5d33e9c7-6d81-485b-bf4a-9a588eda5208
HUMAN PRESCRIPTION DRUG LABEL
Jun 6, 2023
A-S Medication Solutions
DUNS: 830016429
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Pregabalin
PRODUCT DETAILS
INGREDIENTS (11)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PREGABALIN

WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Angioedema
There have been postmarketing reports of angioedema in patients during initial and chronic treatment with pregabalin. Specific symptoms included swelling of the face, mouth (tongue, lips, and gums), and neck (throat and larynx). There were reports of life-threatening angioedema with respiratory compromise requiring emergency treatment. Discontinue pregabalin immediately in patients with these symptoms.
Exercise caution when prescribing pregabalin to patients who have had a previous episode of angioedema. In addition, patients who are taking other drugs associated with angioedema (e.g., angiotensin converting enzyme inhibitors [ACE-inhibitors]) may be at increased risk of developing angioedema.
5.2 Hypersensitivity
There have been postmarketing reports of hypersensitivity in patients shortly after initiation of treatment with pregabalin. Adverse reactions included skin redness, blisters, hives, rash, dyspnea, and wheezing. Discontinue pregabalin immediately in patients with these symptoms.
5.3 Suicidal Behavior and Ideation
Antiepileptic drugs (AEDs), including pregabalin, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Monitor patients treated with any AED for any indication for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
Pooled analyses of 199 placebo-controlled clinical trials (mono-and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted Relative Risk 1.8, 95% CI:1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence rate of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43%, compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number is too small to allow any conclusion about drug effect on suicide.
The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting drug treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed.
The risk of suicidal thoughts or behavior was generally consistent among drugs
in the data analyzed. The finding of increased risk with AEDs of varying
mechanisms of action and across a range of indications suggests that the risk
applies to all AEDs used for any indication. The risk did not vary
substantially by age (5-100 years) in the clinical trials analyzed.
Table 3 shows absolute and relative risk by indication for all evaluated AEDs.
Table 3. Risk by Indication for Antiepileptic Drugs in the Pooled Analysis
|
Indication |
Placebo Patients with Events Per 1000 Patients |
Drug Patients with Events Per 1000 Patients |
Relative Risk: Incidence of Events in Drug Patients/Incidence in Placebo Patients |
Risk Difference: Additional Drug Patients with Events Per 1000 Patients |
|
Epilepsy |
1.0 |
3.4 |
3.5 |
2.4 |
|
Psychiatric |
5.7 |
8.5 |
1.5 |
2.9 |
|
Other |
1.0 |
1.8 |
1.9 |
0.9 |
|
Total |
2.4 |
4.3 |
1.8 |
1.9 |
The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
Anyone considering prescribing pregabalin capsules or any other AED must balance the risk of suicidal thoughts or behavior with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
5.4 Respiratory Depression
There is evidence from case reports, human studies, and animal studies
associating pregabalin with serious, life-threatening, or fatal respiratory
depression when co-administered with central nervous system (CNS) depressants,
including opioids, or in the setting of underlying respiratory impairment.
When the decision is made to co-prescribe pregabalin with another CNS
depressant, particularly an opioid, or to prescribe pregabalin to patients
with underlying respiratory impairment, monitor patients for symptoms of
respiratory depression and sedation, and consider initiating pregabalin at a
low dose. The management of respiratory depression may include close
observation, supportive measures, and reduction or withdrawal of CNS
depressants (including pregabalin).
There is more limited evidence from case reports, animal studies, and human
studies associating pregabalin with serious respiratory depression, without
co-administered CNS depressants or without underlying respiratory impairment.
5.5 Dizziness and Somnolence
Pregabalin may cause dizziness and somnolence. Inform patients that pregabalin-related dizziness and somnolence may impair their ability to perform tasks such as driving or operating machinery [see Patient Counseling Information (17)].
In the pregabalin controlled trials in adult patients, dizziness was
experienced by 30% of pregabalin-treated patients compared to 8% of placebo-
treated patients; somnolence was experienced by 23% of pregabalin-treated
patients compared to 8% of placebo-treated patients. Dizziness and somnolence
generally began shortly after the initiation of pregabalin therapy and
occurred more frequently at higher doses. Dizziness and somnolence were the
adverse reactions most frequently leading to withdrawal (4% each) from
controlled studies. In pregabalin-treated patients reporting these adverse
reactions in short-term, controlled studies, dizziness persisted until the
last dose in 30% and somnolence persisted until the last dose in 42% of
patients [see Drug Interactions (7)].
Pediatric use information is approved for Pfizer’s LYRICA (pregabalin)
Capsules and Oral Solution products. However, due to Pfizer’s marketing
exclusivity rights, this drug product is not labeled with that pediatric
information.
5.6 Increased Risk of Adverse Reactions with Abrupt or Rapid
Discontinuation
As with all antiepileptic drugs (AEDs), withdraw pregabalin gradually to
minimize the potential of increased seizure frequency in patients with seizure
disorders.
Following abrupt or rapid discontinuation of pregabalin, some patients
reported symptoms including insomnia, nausea, headache, anxiety,
hyperhidrosis, and diarrhea.
If pregabalin is discontinued, taper the drug gradually over a minimum of 1
week rather than discontinue the drug abruptly.
5.7 Peripheral Edema
Pregabalin treatment may cause peripheral edema. In short-term trials of
patients without clinically significant heart or peripheral vascular disease,
there was no apparent association between peripheral edema and cardiovascular
complications such as hypertension or congestive heart failure. Peripheral
edema was not associated with laboratory changes suggestive of deterioration
in renal or hepatic function.
In controlled clinical trials in adult patients, the incidence of peripheral
edema was 6% in the pregabalin group compared with 2% in the placebo group. In
controlled clinical trials, 0.5% of pregabalin patients and 0.2% placebo
patients withdrew due to peripheral edema.
Higher frequencies of weight gain and peripheral edema were observed in
patients taking both pregabalin and a thiazolidinedione antidiabetic agent
compared to patients taking either drug alone. The majority of patients using
thiazolidinedione antidiabetic agents in the overall safety database were
participants in studies of pain associated with diabetic peripheral
neuropathy. In this population, peripheral edema was reported in 3% (2/60) of
patients who were using thiazolidinedione antidiabetic agents only, 8%
(69/859) of patients who were treated with pregabalin only, and 19% (23/120)
of patients who were on both pregabalin and thiazolidinedione antidiabetic
agents. Similarly, weight gain was reported in 0% (0/60) of patients on
thiazolidinediones only; 4% (35/859) of patients on pregabalin only; and 7.5%
(9/120) of patients on both drugs.
As the thiazolidinedione class of antidiabetic drugs can cause weight gain
and/or fluid retention, possibly exacerbating or leading to heart failure,
exercise caution when co-administering pregabalin and these agents.
Because there are limited data on congestive heart failure patients with New
York Heart Association (NYHA) Class III or IV cardiac status, exercise caution
when using pregabalin in these patients.
5.8 Weight Gain
Pregabalin treatment may cause weight gain. In pregabalin controlled clinical
trials in adult patients of up to 14 weeks, a gain of 7% or more over baseline
weight was observed in 9% of pregabalin-treated patients and 2% of placebo-
treated patients. Few patients treated with pregabalin (0.3%) withdrew from
controlled trials due to weight gain. Pregabalin associated weight gain was
related to dose and duration of exposure, but did not appear to be associated
with baseline BMI, gender, or age. Weight gain was not limited to patients
with edema [see Warnings and Precautions (5.7)].
Although weight gain was not associated with clinically important changes in
blood pressure in short-term controlled studies, the long-term cardiovascular
effects of pregabalin-associated weight gain are unknown.
Among diabetic patients, pregabalin-treated patients gained an average of 1.6
kg (range: -16 to 16 kg), compared to an average 0.3 kg (range: -10 to 9 kg)
weight gain in placebo patients. In a cohort of 333 diabetic patients who
received Pregabalin for at least 2 years, the average weight gain was 5.2 kg.
While the effects of pregabalin-associated weight gain on glycemic control
have not been systematically assessed, in controlled and longer-term open
label clinical trials with diabetic patients, Pregabalin treatment did not
appear to be associated with loss of glycemic control (as measured by HbA1C).
5.9 Tumorigenic Potential
In standard preclinical in vivo lifetime carcinogenicity studies of pregabalin, an unexpectedly high incidence of hemangiosarcoma was identified in two different strains of mice [see Nonclinical Toxicology (13.1)]. The clinical significance of this finding is unknown. Clinical experience during pregabalin's premarketing development provides no direct means to assess its potential for inducing tumors in humans.
In clinical studies across various patient populations, comprising 6396 patient-years of exposure in patients greater than 12 years of age, new or worsening-preexisting tumors were reported in 57 patients. Without knowledge of the background incidence and recurrence in similar populations not treated with pregabalin, it is impossible to know whether the incidence seen in these cohorts is or is not affected by treatment.
5.10 Ophthalmological Effects
In controlled studies in adult patients, a higher proportion of patients treated with pregabalin reported blurred vision (7%) than did patients treated with placebo (2%), which resolved in a majority of cases with continued dosing. Less than 1% of patients discontinued pregabalin treatment due to vision- related events (primarily blurred vision).
Prospectively planned ophthalmologic testing, including visual acuity testing, formal visual field testing and dilated funduscopic examination, was performed in over 3600 patients. In these patients, visual acuity was reduced in 7% of patients treated with pregabalin and 5% of placebo- treated patients. Visual field changes were detected in 13% of pregabalin-treated, and 12% of placebo- treated patients. Funduscopic changes were observed in 2% of pregabalin- treated and 2% of placebo-treated patients.
Although the clinical significance of the ophthalmologic findings is unknown, inform patients to notify their physician if changes in vision occur. If visual disturbance persists, consider further assessment. Consider more frequent assessment for patients who are already routinely monitored for ocular conditions [see Patient Counseling Information (17)].
5.11 Creatine Kinase Elevations
Pregabalin treatment was associated with creatine kinase elevations. Mean changes in creatine kinase from baseline to the maximum value were 60 U/L for pregabalin-treated patients and 28 U/L for the placebo patients. In all controlled trials in adult patients across multiple patient populations, 1.5% of patients on pregabalin and 0.7% of placebo patients had a value of creatine kinase at least three times the upper limit of normal. Three pregabalin- treated subjects had events reported as rhabdomyolysis in premarketing clinical trials. The relationship between these myopathy events and pregabalin is not completely understood because the cases had documented factors that may have caused or contributed to these events. Instruct patients to promptly report unexplained muscle pain, tenderness, or weakness, particularly if these muscle symptoms are accompanied by malaise or fever. Discontinue treatment with pregabalin if myopathy is diagnosed or suspected or if markedly elevated creatine kinase levels occur.
5.12 Decreased Platelet Count
Pregabalin treatment was associated with a decrease in platelet count. Pregabalin-treated subjects experienced a mean maximal decrease in platelet count of 20 × 103/μL, compared to 11 × 103/μL in placebo patients. In the overall database of controlled trials in adult patients, 2% of placebo patients and 3% of Pregabalin patients experienced a potentially clinically significant decrease in platelets, defined as 20% below baseline value and less than 150 × 103/μL. A single Pregabalin-treated subject developed severe thrombocytopenia with a platelet count less than 20 × 103/ μL. In randomized controlled trials, Pregabalin was not associated with an increase in bleeding- related adverse reactions.
5.13 PR Interval Prolongation
Pregabalin treatment was associated with PR interval prolongation. In analyses of clinical trial ECG data in adult patients, the mean PR interval increase was 3-6 msec at pregabalin doses greater than or equal to 300 mg/day. This mean change difference was not associated with an increased risk of PR increase greater than or equal to 25% from baseline, an increased percentage of subjects with on-treatment PR greater than 200 msec, or an increased risk of adverse reactions of second or third degree AV block.
Subgroup analyses did not identify an increased risk of PR prolongation in patients with baseline PR prolongation or in patients taking other PR prolonging medications. However, these analyses cannot be considered definitive because of the limited number of patients in these categories.
- Angioedema (e.g., swelling of the throat, head and neck) can occur, and may be associated with life-threatening respiratory compromise requiring emergency treatment. Discontinue pregabalin immediately in these cases. (5.1)
- Hypersensitivity reactions (e.g., hives, dyspnea, and wheezing) can occur. Discontinue pregabalin immediately in these patients. (5.2)
- Antiepileptic drugs, including pregabalin, increase the risk of suicidal thoughts or behavior. (5.3)
- Respiratory depression: May occur with pregabalin, when used with concomitant CNS depressants or in the setting of underlying respiratory impairment. Monitor patients and adjust dosage as appropriate. (5.4)
- Pregabalin may cause dizziness and somnolence and impair patients’ ability to drive or operate machinery.(5.5)
- Increased seizure frequency or other adverse reactions may occur if pregabalin is rapidly discontinued. Withdraw pregabalin gradually over a minimum of 1 week. (5.6)
- Pregabalin may cause peripheral edema. Exercise caution when co-administering pregabalin and thiazolidinedione antidiabetic agents. (5.7)
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication
Guide).
Angioedema
Advise patients that pregabalin may cause angioedema, with swelling of the
face, mouth (lip, gum, tongue) and neck (larynx and pharynx) that can lead to
life-threatening respiratory compromise. Instruct patients to discontinue
pregabalin and immediately seek medical care if they experience these symptoms
[ see Warnings and Precautions (5.1)].
Hypersensitivity
Advise patients that pregabalin has been associated with hypersensitivity
reactions such as wheezing, dyspnea, rash, hives, and blisters. Instruct
patients to discontinue pregabalin and immediately seek medical care if they
experience these symptoms [ see Warnings and Precautions (5.2)].
Suicidal Thinking and Behavior
Patients, their caregivers, and families should be counseled that AEDs,
including pregabalin, may increase the risk of suicidal thoughts and behavior
and should be advised of the need to be alert for the emergence or worsening
of symptoms of depression, any unusual changes in mood or behavior, or the
emergence of suicidal thoughts, behavior, or thoughts about self-harm. Report
behaviors of concern immediately to healthcare providers [ see Warnings and Precautions (5.3)].
Respiratory Depression
Inform patients about the risk of respiratory depression. Include information
that the risk is greatest for those using concomitant central nervous system
(CNS) depressants (such as opioid analgesics) or in those with underlying
respiratory impairment. Teach patients how to recognize respiratory depression
and advise them to seek medical attention immediately if it occurs [see Warnings and Precautions (5.4)].
Dizziness and Somnolence
Counsel patients that pregabalin may cause dizziness, somnolence, blurred
vision and other CNS signs and symptoms. Accordingly, advise patients not to
drive, operate complex machinery, or engage in other hazardous activities
until they have gained sufficient experience on pregabalin to gauge whether or
not it affects their mental, visual, and/or motor performance adversely. [ see Warnings and Precautions (5.5)].CNS Depressants
Inform patients who require concomitant treatment with central nervous system
depressants such as opiates or benzodiazepines that they may experience
additive CNS side effects, such as respiratory depression, somnolence, and
dizziness [seeWarnings and Precautions (5.4,5.5) and Drug Interactions (7)].Advise patients to avoid consuming alcohol while taking pregabalin, as
pregabalin may potentiate the impairment of motor skills and sedating effects
of alcohol.
Adverse Reactions with Abrupt or Rapid Discontinuation
Advise patients to take pregabalin as prescribed. Abrupt or rapid
discontinuation may result in increased seizure frequency in patients with
seizure disorders, and insomnia, nausea, headache, anxiety, hyperhidrosis, or
diarrhea [see Warnings and Precautions (5.6)].
Missed Dose
Counsel patients if they miss a dose, they should take it as soon as they
remember. If it is almost time for the next dose, they should skip the missed
dose and take the next dose at their regularly scheduled time. Instruct
patients not to take two doses at the same time.
Weight Gain and Edema
Counsel patients that pregabalin may cause edema and weight gain. Advise
patients that concomitant treatment with pregabalin and a thiazolidinedione
antidiabetic agent may lead to an additive effect on edema and weight gain.
For patients with preexisting cardiac conditions, this may increase the risk
of heart failure. [ see Warnings and Precautions (5.7 and 5.8)].
Ophthalmological Effects
Counsel patients that pregabalin capsule may cause visual disturbances. Inform
patients that if changes in vision occur, they should notify their physician [ see Warnings and Precautions (5.10)].
Creatine Kinase Elevations
Instruct patients to promptly report unexplained muscle pain, tenderness, or
weakness, particularly if accompanied by malaise or fever. [ see Warnings and Precautions (5.11)].
Pregnancy
There is a pregnancy exposure registry that monitors pregnancy outcomes in
women exposed to pregabalin during pregnancy [ see Use in Specific Populations (8.1)].
Lactation
Advise nursing mothers that breastfeeding is not recommended during treatment
with pregabalin [ see Use in Specific Populations (8.2)].
Male Fertility
Inform men being treated with pregabalin who plan to father a child of the
potential risk of male-mediated teratogenicity. In preclinical studies in
rats, pregabalin was associated with an increased risk of male-mediated
teratogenicity. The clinical significance of this finding is uncertain [ see Nonclinical Toxicology (13.1)and Use in specific populations (8.3)].
Dermatopathy
Instruct diabetic patients to pay particular attention to skin integrity while
being treated with pregabalin and to inform their healthcare provider about
any sores or skin problems. Some animals treated with pregabalin developed
skin ulcerations, although no increased incidence of skin lesions associated
with pregabalin was observed in clinical trials [ see Nonclinical Toxicology (13.2)].
Manufactured by:
** MSN Laboratories Private Limited**
****Telangana – 509 228, INDIA
** Distributed by:**
** Novadoz Pharmaceuticals LLC**
Piscataway, NJ 08854-3714
Issued on:
June 2020
DRUG ABUSE AND DEPENDENCE SECTION
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
Pregabalin is a Schedule V controlled substance.
Pregabalin is not known to be active at receptor sites associated with drugs of abuse. As with any CNS active drug, carefully evaluate patients for history of drug abuse and observe them for signs of pregabalin misuse or abuse (e.g., development of tolerance, dose escalation, drug-seeking behavior).
9.2 Abuse
In a study of recreational users (N=15) of sedative/hypnotic drugs, including alcohol, pregabalin (450 mg, single dose) received subjective ratings of "good drug effect," "high" and "liking" to a degree that was similar to diazepam (30 mg, single dose). In controlled clinical studies in over 5500 patients, 4 % of pregabalin -treated patients and 1 % of placebo-treated patients overall reported euphoria as an adverse reaction, though in some patient populations studied, this reporting rate was higher and ranged from 1 to 12%.
9.3 Dependence
In clinical studies, following abrupt or rapid discontinuation of pregabalin, some patients reported symptoms including insomnia, nausea, headache or diarrhea [see Warnings and Precautions (5.3)], consistent with physical dependence. In the postmarketing experience, in addition to these reported symptoms there have also been reported cases of anxiety and hyperhidrosis.
DESCRIPTION SECTION
11 DESCRIPTION
Pregabalin is described chemically as (S)-3-(aminomethyl)-5-methylhexanoic
acid. The molecular formula is C8H17NO2and the molecular weight is 159.23. The
chemical structure of pregabalin is:
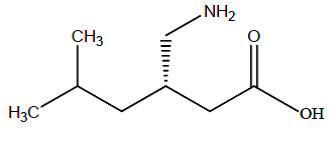
Pregabalin is a white to off-white, crystalline solid with a pKa1of 4.2 and a
pKa2of 10.6. It is freely soluble in water and both basic and acidic aqueous
solutions. The log of the partition coefficient (n-octanol/0.05M phosphate
buffer) at pH 7.4 is – 1.35.
Pregabalin capsules are administered orally and are supplied as imprinted
hard-shell capsules containing 25, 50, 75, 100, 150, 200, 225, and 300 mg of
pregabalin, along with mannitol and talc as inactive ingredients. The capsule
shells contain FD&C Blue No. 2, gelatin, titanium dioxide, sodium lauryl
sulfate and is imprinted with black ink. Black imprinting ink contains
shellac, propylene glycol, black iron oxide and potassium hydroxide.
