IRESSA
These highlights do not include all the information needed to use IRESSA safely and effectively. See full prescribing information for IRESSA.IRESSA (gefitinib) tablets for oral useInitial U.S. Approval: 2015
827d60e8-7e07-41b7-c28b-49ef1c4a5a41
HUMAN PRESCRIPTION DRUG LABEL
Feb 28, 2023
AstraZeneca Pharmaceuticals LP
DUNS: 054743190
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Gefitinib
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
Drug Labeling Information
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
IRESSA is indicated for the first-line treatment of patients with metastatic non-small cell lung cancer (NSCLC) whose tumors have epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 (L858R) substitution mutations as detected by an FDA-approved test [see Clinical Studies (14)].
Limitation of Use: Safety and efficacy of IRESSA have not been established in patients with metastatic NSCLC whose tumors have EGFR mutations other than exon 19 deletions or exon 21 (L858R) substitution mutations [see Clinical Studies (14)].
IRESSA is a tyrosine kinase inhibitor indicated for the first-line treatment of patients with metastatic non-small cell lung cancer (NSCLC) whose tumors have epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 (L858R) substitution mutations as detected by an FDA-approved test. (1)
Limitation of Use: Safety and efficacy of IRESSA have not been established in patients whose tumors have EGFR mutations other than exon 19 deletions or exon 21 (L858R) substitution mutations. (1)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Interstitial Lung Disease (ILD)
ILD or ILD-like adverse drug reactions (e.g., lung infiltration, pneumonitis, acute respiratory distress syndrome, or pulmonary fibrosis) occurred in 1.3% of the 2462 patients who received IRESSA across clinical trials; of these, 0.7% were Grade 3 or higher and 3 cases were fatal.
Withhold IRESSA and promptly investigate for ILD in any patient who presents with worsening of respiratory symptoms such as dyspnea, cough and fever. Permanently discontinue IRESSA if ILD is confirmed [see Dosage and Administration (2.4), Adverse Reactions (6.1)].
5.2 Hepatotoxicity
In patients who received IRESSA across clinical trials, 11.4% of patients had increased alanine aminotransferase (ALT), 7.9% of patients had increased aspartate aminotransferase (AST), and 2.7% of patients had increased bilirubin. Grade 3 or higher liver test abnormalities occurred in 5.1% (ALT), 3.0% (AST), and 0.7% (bilirubin) of patients. The incidence of fatal hepatotoxicity was 0.04%.
Obtain periodic liver function testing. Withhold IRESSA in patients with worsening liver function and discontinue in patients with severe hepatic impairment [see Dosage and Administration (2.4), Adverse Reactions (6.1), Use in Specific Populations (8.7)].
5.3 Gastrointestinal Perforation
Gastrointestinal perforation occurred in three (0.1%) of the 2462 IRESSA- treated patients across clinical trials [see Adverse Reactions (6.1)]. Permanently discontinue IRESSA in patients who develop gastrointestinal perforation [see Dosage and Administration (2.4)].
5.4 Severe or Persistent Diarrhea
Grade 3 or 4 diarrhea occurred in 3% of 2462 IRESSA-treated patients across clinical trials. Withhold IRESSA for severe or persistent (up to 14 days) diarrhea [see Dosage and Administration (2.4), Adverse Reactions (6.1)].
5.5 Ocular Disorders including Keratitis
Ocular disorders [keratitis (0.1%), corneal erosion and aberrant eyelash growth (0.2%), conjunctivitis, blepharitis and dry eye (6.7%)] occurred in the 2462 IRESSA-treated patients across clinical trials. The incidence of Grade 3 ocular disorders was 0.1% [see Adverse Reactions (6.1)]. Interrupt or discontinue IRESSA for severe, or worsening ocular disorders [see Dosage and Administration (2.4)].
5.6 Bullous and Exfoliative Skin Disorders
Bullous conditions including toxic epidermal necrolysis, Stevens Johnson syndrome and erythema multiforme have been reported from treatment with IRESSA. Erythema multiforme and dermatitis bullous have been reported in two patients (0.08%) across NSCLC trials (Study 2, Study 3 and Study 4). IRESSA treatment should be interrupted or discontinued if the patient develops severe bullous, blistering or exfoliating conditions.
5.7 Embryo-fetal Toxicity
Based on its mechanism of action and data from animal reproduction studies IRESSA can cause fetal harm when administered to a pregnant woman. In animal reproductive studies, oral administration of gefitinib from organogenesis through weaning resulted in fetotoxicity and neonatal death at doses below the recommended human dose. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with IRESSA and for at least two weeks following completion of therapy [see Use in Specific Populations (8.1, 8.3)].
•
Interstitial lung disease (ILD): ILD occurred in patients taking IRESSA. Withhold IRESSA for worsening of respiratory symptoms. Discontinue IRESSA if ILD is confirmed. (2.4, 5.1)
•
Hepatotoxicity: Obtain periodic liver function testing. Withhold IRESSA for Grade 2 or higher for ALT and/or AST elevations. Discontinue for severe hepatic impairment. (2.4, 5.2)
•
Gastrointestinal perforation: Discontinue IRESSA for gastrointestinal perforation. (2.4, 5.3)
•
Diarrhea: Withhold IRESSA for Grade 3 or higher diarrhea. (2.4, 5.4)
•
Ocular Disorders including Keratitis: Withhold IRESSA for signs and symptoms of severe or worsening ocular disorders including keratitis. Discontinue for persistent ulcerative keratitis. (2.4, 5.5)
•
Bullous and Exfoliative Skin Disorders: Withhold IRESSA for Grade 3 or higher skin reactions or exfoliative conditions. (2.4, 5.6)
•
Embryo-fetal Toxicity: Can cause fetal harm. Advise of potential risk to a fetus and use of effective contraception. (5.7, 8.1, 8.3)
DESCRIPTION SECTION
11 DESCRIPTION
Gefitinib is a kinase inhibitor.
The chemical name of gefitinib is 4-Quinazolinamine N-(3-chloro-4-fluorophenyl)-7-methoxy-6-[3-(4-morpholinyl) propoxy] and the following structural formula:
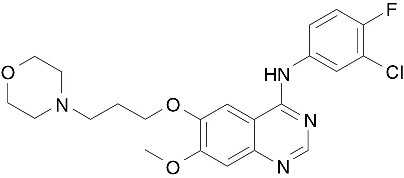
Gefitinib has the molecular formula C22H24ClFN4O3, a relative molecular mass of 446.9 daltons and is a white-colored powder. Gefitinib is a free base. The molecule has pKas of 5.4 and 7.2. Gefitinib can be defined as sparingly soluble at pH 1, but is practically insoluble above pH 7, with the solubility decreasing sharply between pH 4 and pH 6. In non-aqueous solvents, gefitinib is freely soluble in glacial acetic acid and dimethyl sulfoxide, soluble in pyridine, sparingly soluble in tetrahydrofuran, and slightly soluble in methanol, ethanol (99.5%), ethyl acetate, propan-2-ol and acetonitrile.
IRESSA® (gefitinib) tablets are available as brown film-coated tablets, containing 250 mg of gefitinib, for oral administration. The inactive ingredients of the tablet core of IRESSA tablets are lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, povidone, sodium lauryl sulfate and magnesium stearate. The tablet coating is composed of hypromellose, polyethylene glycol 300, titanium dioxide, red ferric oxide and yellow ferric oxide.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
IRESSA® (gefitinib) is available as 250 mg tablets.
IRESSA 250 mg tablets are round, biconvex, brown film-coated, debossed with "IRESSA 250" on one side and plain on the other side.
IRESSA® (gefitinib) tablets are supplied as:
Bottles of 30 Tablets (NDC 0310-0482-30)
Store at controlled room temperature 20°C-25°C (68°F-77°F) [see USP Controlled Room Temperature].
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labelling (Patient Information).
Interstitial Lung Disease: Advise patients to immediately contact their healthcare provider for new onset or worsening of pulmonary symptoms such as dyspnea, cough and fever [see Warnings and Precautions (5.1)].
Hepatotoxicity: Inform patients that they will need to undergo lab tests to monitor for liver function. Advise patients to contact their healthcare provider to report any new symptoms indicating hepatic toxicity [see Warnings and Precautions (5.2)].
Gastrointestinal Perforation: Advise patients that IRESSA can increase the risk of gastrointestinal perforation and to seek immediate medical attention for severe abdominal pain [see Warnings and Precautions (5.3)].
Severe or Persistent Diarrhea: Advise patients to contact their healthcare provider for severe or persistent diarrhea [see Warnings and Precautions (5.4)].
Ocular Disorders including Keratitis: Advise patients promptly to contact their healthcare provider if they develop eye symptoms, lacrimation, light sensitivity, blurred vision, eye pain, red eye or changes in vision [see Warnings and Precautions (5.5)].
Bullous and Exfoliative Skin Disorders: Advise patients that IRESSA can increase the risk of bullous and exfoliative skin disorders and to seek immediately medical attention for severe skin reactions [see Warnings and Precautions (5.6)].
Embryo-fetal Toxicity: Advise pregnant women of the potential risk to a fetus or potential risk for loss of the pregnancy [see Warnings and Precautions (5.7), Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment with IRESSA and for at least two weeks following completion of therapy [see Use in Specific Populations (8.3)].
Lactation: Advise women to discontinue breast-feeding during treatment with IRESSA [see Use in Specific Populations (8.2)].
IRESSA is a trademark of the AstraZeneca group of companies.
©AstraZeneca 2023
Distributed by:
AstraZeneca Pharmaceuticals LP
Wilmington, DE 19850
