Tranexamic Acid
These highlights do not include all the information needed to use TRANEXAMIC ACID INJECTION safely and effectively. See full prescribing information for TRANEXAMIC ACID INJECTION. TRANEXAMIC ACID injection, for intravenous use Initial U.S. Approval: 1986
a93d6ef3-e160-db0d-142f-9b91af8d8428
HUMAN PRESCRIPTION DRUG LABEL
Sep 22, 2025
Apotex Corp.
DUNS: 845263701
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
tranexamic acid
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (2)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 10 Vial Carton Label
Each vial contains 1 g tranexamic acid, USP.
Store at 25°C (77°F); excursions permitted to 15°C - 30°C (59°F -86°F).
Usual dosage:
See package insert
Manufactured by:
Indoco Remedies Limited
L-32, 33, 34, Verna Industrial Area,
Verna, Goa-403722,
India
Manufactured for:
Apotex Corp.
Weston, FL 33326
10 x 10 mL Vials
NDC 60505-6169-1
Tranexamic Acid Injection, USP
1,000 mg/10 mL
(100 mg/mL)
Solution for intravenous injection
Single-Dose ONLY
Discard any remaining portion after
single use
Rx only

DESCRIPTION SECTION
11 DESCRIPTION
Tranexamic acid, USP is trans-4-(aminomethyl)cyclohexanecarboxylic acid, an antifibrinolytic agent. Tranexamic acid, USP is a white or almost white crystalline powder. The structural formula is
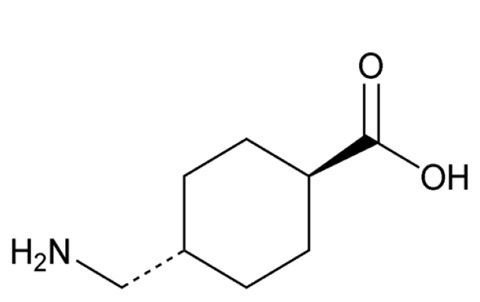
Molecular Formula: C8H15NO2
Molecular Weight: 157.21 g/mol
Each mL of the sterile solution for intravenous injection contains 100 mg tranexamic acid, USP and water for injection, USP to 1 mL. The aqueous solution for injection has a pH of 6.5 to 8.0.
