fluvoxamine maleate
These highlights do not include all the information needed to use FLUVOXAMINE MALEATE TABLETS safely and effectively. See full prescribing information for FLUVOXAMINE MALEATE TABLETS. FLUVOXAMINE MALEATE tablets, for oral useInitial U.S. Approval: 1994
ddc9e7e8-f041-43bb-970b-cd4b30367637
HUMAN PRESCRIPTION DRUG LABEL
Nov 22, 2023
A-S Medication Solutions
DUNS: 830016429
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Fluvoxamine maleate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (19)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FLUVOXAMINE MALEATE

DESCRIPTION SECTION
11 DESCRIPTION
Fluvoxamine maleate is a selective serotonin (5-HT) reuptake inhibitor (SSRI) belonging to the chemical series, the 2-aminoethyl oxime ethers of aralkylketones.
It is chemically designated as 5-methoxy-4′-(trifluoromethyl)valerophenone-(E)-O-(2-aminoethyl)oxime maleate (1:1) and has the empirical formula C15H21O2N2F3∙C4H4O4. Its molecular weight is 434.41.
The structural formula is:
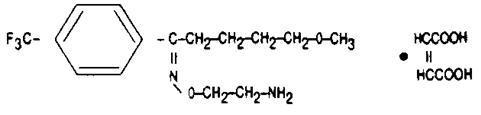
Fluvoxamine maleate is a white to off white, odorless, crystalline powder which is sparingly soluble in water, freely soluble in ethanol and chloroform and practically insoluble in diethyl ether.
Fluvoxamine Maleate Tablets are available in 25 mg, 50 mg and 100 mg strengths for oral administration. In addition to the active ingredient, fluvoxamine maleate, each tablet contains the following inactive ingredients: mannitol, polyethylene glycol, polyvinyl alcohol, pregelatinized starch (potato), silicon dioxide, sodium stearyl fumarate, starch (corn), talc, and titanium dioxide. The 50 mg and 100 mg tablets also contain synthetic iron oxides.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Product: 50090-5988
NDC: 50090-5988-0 90 TABLET, COATED in a BOTTLE
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Prescribers or other health professionals should inform patients, their families, and their caregivers about the benefits and risks associated with treatment with Fluvoxamine Maleate Tablets and should counsel them in the appropriate use. A patient Medication Guide about “Antidepressant Medicines, Depression and other Serious Mental Illnesses, and Suicidal Thoughts or Actions” is available for Fluvoxamine Maleate Tablets. The prescriber or health professional should instruct patients, their families, and their caregivers to read the Medication Guide and should assist them in understanding its contents. Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions they may have. The complete text of the Medication Guide is reprinted at the end of this document.
Patients should be advised of the following issues and asked to alert their prescriber if these occur while taking Fluvoxamine Maleate Tablets.
Clinical Worsening and Suicide Risk
Patients, their families, and their caregivers should be encouraged to be alert to the emergence of anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, mania, other unusual changes in behavior, worsening of depression, and suicidal ideation, especially early during antidepressant treatment and when the dose is adjusted up or down. Families and caregivers of patients should be advised to look for the emergence of such symptoms on a day-to-day basis, since changes may be abrupt. Such symptoms should be reported to the patient’s prescriber or health professional, especially if they are severe, abrupt in onset, or were not part of the patient’s presenting symptoms. Symptoms such as these may be associated with an increased risk for suicidal thinking and behavior and indicate the need for very close monitoring and possibly changes in the medication [see Boxed Warning, Warnings and Precautions (5.1)].
Serotonin Syndrome
Patients should be cautioned about the risk of serotonin syndrome particularly with the concomitant use of fluvoxamine with other serotonergic agents (including triptans, tricyclic antidepressants, opioids, lithium, tryptophan, buspirone, amphetamines, and St. John's Wort) [see Warnings and Precautions (5.2)].
Angle Closure Glaucoma
Patients should be advised that taking Fluvoxamine Maleate Tablets can cause mild pupillary dilation, which in susceptible individuals, can lead to an episode of angle closure glaucoma. Pre-existing glaucoma is almost always open-angle glaucoma because angle closure glaucoma, when diagnosed, can be treated definitively with iridectomy. Open-angle glaucoma is not a risk factor for angle closure glaucoma. Patients may wish to be examined to determine whether they are susceptible to angle closure, and have a prophylactic procedure (e.g., iridectomy), if they are susceptible [see Warnings and Precautions (5.3)].
Interference with Cognitive or Motor Performance
Since any psychoactive drug may impair judgment, thinking, or motor skills, patients should be cautioned about operating hazardous machinery, including automobiles, until they are certain that Fluvoxamine Maleate Tablets therapy does not adversely affect their ability to engage in such activities.
Pregnancy
•
Advise pregnant women to notify their healthcare provider if they become pregnant or intend to become pregnant during treatment with Fluvoxamine Maleate Tablets.
•
Advise patients that Fluvoxamine Maleate Tablets use late in pregnancy may lead to an increased risk for neonatal complications requiring prolonged hospitalization, respiratory support, tube feeding, and/or persistent pulmonary hypertension of the newborn (PPHN).
•
Advise women that there is a risk of relapse with discontinuation of antidepressants.
•
Advise women that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to Fluvoxamine Maleate Tablets during pregnancy [see Use in Specific Populations (8.1)].
Lactation
Advise breastfeeding women using Fluvoxamine Maleate Tablets to monitor infants for diarrhea, vomiting, decreased sleep, and agitation and to seek medical care if they notice these signs [see Use in Specific Populations (8.2)].
Concomitant Medication
Patients should be advised to notify their physicians if they are taking, or plan to take, any prescription or over-the-counter drugs, since there is a potential for clinically important interactions with Fluvoxamine Maleate Tablets.
Patients should be cautioned about the concomitant use of fluvoxamine and NSAIDs, aspirin, or other drugs that affect coagulation since the combined use of psychotropic drugs that interfere with serotonin reuptake and these agents has been associated with an increased risk of bleeding [see Warnings and Precautions (5.8)].
Because of the potential for the increased risk of serious adverse reactions including severe lowering of blood pressure and sedation when fluvoxamine and tizanidine are used together, fluvoxamine should not be used with tizanidine [see Warnings and Precautions (5.5)].
Because of the potential for the increased risk of serious adverse reactions when fluvoxamine and alosetron are used together, fluvoxamine should not be used with LotronexTM (alosetron) [see Warnings and Precautions (5.7)].
Sexual Dysfunction
Advise patients that use of Fluvoxamine Maleate Tablets may cause symptoms of sexual dysfunction in both male and female patients. Inform patients that they should discuss any changes in sexual function and potential management strategies with their healthcare provider [see Warnings and Precautions (5.16)].
Alcohol
As with other psychotropic medications, patients should be advised to avoid alcohol while taking Fluvoxamine Maleate Tablets.
Allergic Reactions
Patients should be advised to notify their physicians if they develop a rash, hives, or a related allergic phenomenon during therapy with Fluvoxamine Maleate Tablets.
All brand names listed are the registered trademarks of their respective owners and are not trademarks of ANI Pharmaceuticals, Inc.
Manufactured by:
ANI Pharmaceuticals, Inc.
Baudette, MN 56623

