lurasidone hydrochloride
These highlights do not include all the information needed to use LURASIDONE HYDROCHLORIDE TABLETS safely and effectively. See full prescribing information for LURASIDONE HYDROCHLORIDE TABLETS. LURASIDONE HYDROCHLORIDE tablets, for oral use Initial U.S. Approval: 2010
36b6bacd-ac11-493b-a8a4-8dad448ed9d5
HUMAN PRESCRIPTION DRUG LABEL
Jan 6, 2023
Ascend Laboratories, LLC
DUNS: 141250469
Products 5
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
lurasidone hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
lurasidone hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
lurasidone hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
lurasidone hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (13)
lurasidone hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC: 67877-638-30
**** Lurasidone Hydrochloride Tablets 20 mg****
**** 30 Tablets****
NDC: 67877-639-90
** Lurasidone Hydrochloride Tablets 40 mg**
** 90 Tablets**

NDC: 67877-640-30
** Lurasidone Hydrochloride Tablets 60 mg**
** 30 Tablets**
**
**
** NDC: 67877-641-38**
** Lurasidone Hydrochloride Tablets 80 mg**
** Carton of 100 (10X10) Unit dose Tablets**
NDC: 67877-642-05
**** Lurasidone Hydrochloride Tablets 120 mg****
**** 500 Tablets****

DRUG ABUSE AND DEPENDENCE SECTION
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
Lurasidone hydrochloride tablets are not controlled substance.
9.2 Abuse
Lurasidone hydrochloride tablets have not been systematically studied in humans for its potential for abuse or physical dependence or its ability to induce tolerance. While clinical studies with lurasidone hydrochloride tablets did not reveal any tendency for drug-seeking behavior, these observations were not systematic and it is not possible to predict the extent to which a CNS- active drug will be misused, diverted and/or abused once it is marketed. Patients should be evaluated carefully for a history of drug abuse, and such patients should be observed carefully for signs of lurasidone hydrochloride tablets misuse or abuse (e.g., development of tolerance, drug-seeking behavior, increases in dose).
OVERDOSAGE SECTION
10 OVERDOSAGE
10.1 Human Experience
In premarketing clinical studies, accidental or intentional overdosage of lurasidone hydrochloride tablets were identified in one patient who ingested an estimated 560 mg of lurasidone hydrochloride tablets. This patient recovered without sequelae. This patient resumed lurasidone hydrochloride tablets treatment for an additional two months.
10.2 Management of Overdosage
No specific antidotes for lurasidone hydrochloride tablets are known. In managing overdose, provide supportive care, including close medical supervision and monitoring, and consider the possibility of multiple drug involvement. If an overdose occurs, consult a Certified Poison Control Center (1-800-222-1222 or www.poison.org).
Cardiovascular monitoring should commence immediately, including continuous electrocardiographic monitoring for possible arrhythmias. If antiarrhythmic therapy is administered, disopyramide, procainamide, and quinidine carry a theoretical hazard of additive QT-prolonging effects when administered in patients with an acute overdose of lurasidone hydrochloride tablets. Similarly, the alpha-blocking properties of bretylium might be additive to those of lurasidone hydrochloride tablets, resulting in problematic hypotension.
Hypotension and circulatory collapse should be treated with appropriate measures. Epinephrine and dopamine should not be used, or other sympathomimetics with beta-agonist activity, since beta stimulation may worsen hypotension in the setting of lurasidone hydrochloride tablets -induced alpha blockade. In case of severe extrapyramidal symptoms, anticholinergic medication should be administered.
Gastric lavage (after intubation if patient is unconscious) and administration of activated charcoal together with a laxative should be considered.
The possibility of obtundation, seizures, or dystonic reaction of the head and neck following overdose may create a risk of aspiration with induced emesis.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of lurasidone in the treatment of bipolar depression is unclear. However, its efficacy in bipolar depression could be mediated through a combination of central dopamine D2 and serotonin Type 2 (5HT2A) receptor antagonism.
12.2 Pharmacodynamics
Lurasidone is an antagonist with high affinity binding at the dopamine D2 receptors (Ki of 1 nM) and the serotonin 5-HT2A (Ki of 0.5 nM) and 5-HT7 (Ki of 0.5 nM) receptors. It also binds with moderate affinity to the human α2C adrenergic receptors (Ki of 11 nM), is a partial agonist at serotonin 5-HT1A (Ki of 6.4 nM) receptors, and is an antagonist at the α2A adrenergic receptors (Ki of 41 nM). Lurasidone exhibits little or no affinity for histamine H1 and muscarinic M1 receptors (IC50> 1,000 nM).
ECG Changes
The effects of lurasidone hydrochloride tablets on the QTc interval were evaluated in a randomized, double-blind, multiple-dose, parallel-dedicated thorough QT study in 43 patients with another indication, who were treated with lurasidone hydrochloride tablets doses of 120 mg daily, 600 mg daily and completed the study. The maximum mean (upper 1-sided, 95% CI) increase in baseline- adjusted QTc intervals based on individual correction method (QTcI) was 7.5 (11.7) ms and 4.6 (9.5) ms, for the 120 mg and 600 mg dose groups respectively, observed at 2 to 4 hours after dosing. In this study, there was no apparent dose (exposure)-response relationship.
In short-term, placebo-controlled studies in bipolar depression and another indication, no post- baseline QT prolongations exceeding 500 msec were reported in patients treated with lurasidone hydrochloride tablets or placebo.
12.3 Pharmacokinetics
Adults
The activity of lurasidone hydrochloride tablets are primarily due to the parent drug. The pharmacokinetics of lurasidone hydrochloride tablets are dose-proportional within a total daily dose range of 20 mg to 160 mg. Steady- state concentrations of lurasidone hydrochloride tablets are reached within 7 days of starting lurasidone hydrochloride tablets.
Following administration of 40 mg of lurasidone hydrochloride tablets, the mean (%CV) elimination half-life was 18 (7) hours.
Absorption and Distribution: Lurasidone hydrochloride tablets are absorbed and reaches peak serum concentrations in approximately 1-3 hours. It is estimated that 9-19% of an administered dose is absorbed. Following administration of 40 mg of lurasidone hydrochloride tablets, the mean (%CV) apparent volume of distribution was 6173 (17.2) L. Lurasidone hydrochloride tablets is highly bound (~99%) to serum proteins.
In a food effect study, lurasidone hydrochloride tablets mean Cmax and AUC were about 3-times and 2-times, respectively, when administered with food compared to the levels observed under fasting conditions. Lurasidone hydrochloride tablets exposure was not affected as meal size was increased from 350 to 1000 calories and was independent of meal fat content [see Dosage and Administration (2.3)].
In clinical studies, establishing the safety and efficacy of lurasidone hydrochloride tablets, patients were instructed to take their daily dose with food [see Dosage and Administration (2.3)].
Metabolism and Elimination: Lurasidone hydrochloride tablets are metabolized mainly via CYP3A4. The major biotransformation pathways are oxidative N-dealkylation, hydroxylation of norbornane ring, and S-oxidation. Lurasidone hydrochloride tablets are metabolized into two active metabolites (ID-14283 and ID-14326) and two major non-active metabolites (ID-20219 and ID-20220). Based on in vitro studies, lurasidone hydrochloride tablets are not a substrate of CYP1A1, CYP1A2, CYP2A6, CYP4A11, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6 or CYP2E1 enzymes. Because lurasidone hydrochloride tablets are not a substrate for CYP1A2, smoking is not expected to have an effect on the pharmacokinetics of lurasidone hydrochloride tablets.
Transporter proteins: In vitro studies suggest lurasidone hydrochloride tablets are not a substrate of OATP1B1 or OATP1B3, however, is probably a substrate of P-gp and BCRP. In vitro studies indicate that lurasidone hydrochloride tablets are not expected to inhibit transporters OATP1B1, OATP1B3, OCT1, OCT2, OAT1, OAT3, MATE1, MATE2-K and BSEP at clinically relevant concentrations. Lurasidone hydrochloride tablets are not a clinically significant inhibitor of P-gp. However, it may inhibit BCRP.
Total excretion of radioactivity in urine and feces combined was approximately 89%, with about 80% recovered in feces and 9% recovered in urine, after a single dose of [14C]-labeled lurasidone hydrochloride tablets.
Following administration of 40 mg of lurasidone hydrochloride tablets, the mean (%CV) apparent clearance was 3902 (18.0) mL/min.
Drug Interaction Studies
Effects of other drugs on the exposure of lurasidone are summarized in Figure 5. A population PK analyses concluded that coadministration of lithium 300-2400 mg/day or valproate 300-2000 mg/day with lurasidone for up to 6 weeks has minimal effect on lurasidone exposure.
And the effects of lurasidone hydrochloride tablets on the exposures of other drugs are summarized in Figure 2. A population PK analyses concluded that coadministration of lurasidone has minimal effect on lithium and valproate exposure when it is coadministered with lithium 300-2400 mg/day or valproate 300-2000 mg/day.
Figure 1: Impact of Other Drugs on Lurasidone Hydrochloride Tablets
Pharmacokinetics
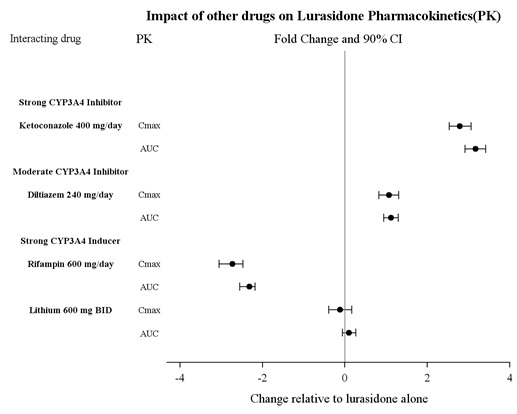
Figure 2: Impact of Lurasidone Hydrochloride Tablets on Other Drugs
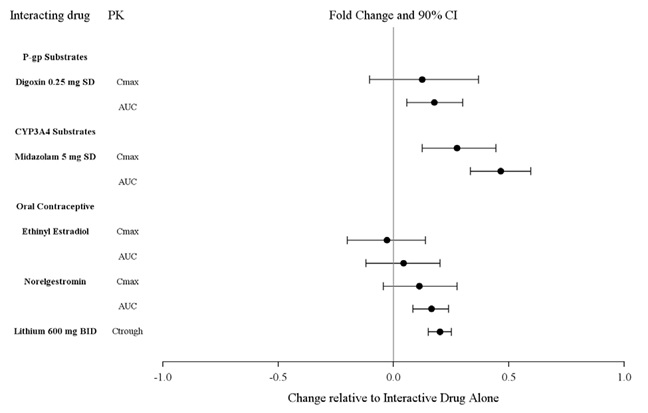
Studies in Specific Populations
The effect of intrinsic patient factors on the pharmacokinetics of lurasidone
hydrochloride tablets are presented in Figure 3.
Pediatric Patients
Lurasidone hydrochloride tablets exposure (i.e., steady-state Cmax and AUC) in
children and adolescent patients (10 to 17 years of age) was generally similar
to that in adults across the dose range from 40 to 160 mg, without adjusting
for body weight.
Figure 3: Impact of Other Patient Factors on Lurasidone Hydrochloride
Tablets Pharmacokinetics
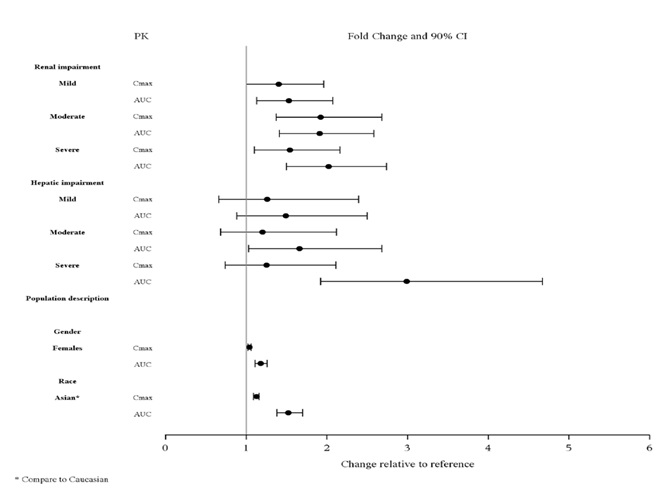
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis: Lurasidone increased incidences of malignant mammary gland tumors and pituitary gland adenomas in female mice orally dosed with 30, 100, 300, or 650 mg/kg/day. The lowest dose produced plasma levels (AUC) approximately equal to those in humans receiving the MRHD of 160 mg/day. No increases in tumors were seen in male mice up to the highest dose tested, which produced plasma levels (AUC) 14 times those in humans receiving the MRHD.
Lurasidone increased the incidence of mammary gland carcinomas in female rats orally dosed at 12 and 36 mg/kg/day: the lowest dose; 3 mg/kg/day is the no- effect dose which produced plasma levels (AUC) 0.4 times those in humans receiving the MRHD. No increases in tumors were seen in male rats up to the highest dose tested, which produced plasma levels (AUC) 6 times those in humans receiving the MRHD.
Proliferative and/or neoplastic changes in the mammary and pituitary glands of rodents have been observed following chronic administration of antipsychotic drugs and are considered to be prolactin-mediated [see Warnings and Precautions (5.7)].
Mutagenesis: Lurasidone did not cause mutation or chromosomal aberration when tested in vitro and in vivo test battery. Lurasidone was negative in the Ames gene mutation test, the Chinese Hamster Lung (CHL) cells, and in the in vivo mouse bone marrow micronucleus test up to 2000 mg/kg which is 61 times the MRHD of 160 mg/day based on mg/m2 body surface area.
Impairment of Fertility: Estrus cycle irregularities were seen in rats orally administered lurasidone at 1.5, 15 and 150 mg/kg/day for 15 consecutive days prior to mating, during the mating period, and through gestation day 7. No effect was seen at the lowest dose of 0.1 mg/kg which is approximately 0.006 times the MRHD of 160 mg/day based on mg/m2. Fertility was reduced only at the highest dose, which was reversible after a 14 day drug-free period. The no- effect dose for reduced fertility was approximately equal to the MRHD based on mg/m2.
Lurasidone had no effect on fertility in male rats treated orally for 64 consecutive days prior to mating and during the mating period at doses up to 9 times the MRHD based on mg/m2.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.2 Depressive Episodes Associated with Bipolar I Disorder
Adults
Monotherapy
The efficacy of lurasidone hydrochloride tablets, as monotherapy, was established in a 6-week, multicenter, randomized, double-blind, placebo- controlled study of adult patients (mean age of 41.5 years, range 18 to 74) who met DSM-IV-TR criteria for major depressive episodes associated with bipolar I disorder, with or without rapid cycling, and without psychotic features (N=485). Patients were randomized to one of two flexible-dose ranges of lurasidone hydrochloride tablets (20 to 60 mg/day, or 80 to 120 mg/day) or placebo.
The primary rating instrument used to assess depressive symptoms in this study was the Montgomery-Asberg Depression Rating Scale (MADRS), a 10-item clinician-rated scale with total scores ranging from 0 (no depressive features) to 60 (maximum score). The primary endpoint was the change from baseline in MADRS score at Week 6. The key secondary instrument was the Clinical Global Impression-Bipolar-Severity of Illness scale (CGI-BP-S), a clinician-rated scale that measures the subject’s current illness state on a 7-point scale, where a higher score is associated with greater illness severity.
For both dose groups, lurasidone hydrochloride tablets was superior to placebo in reduction of MADRS and CGI-BP-S scores at Week 6. The primary efficacy results are provided in Table 37. The high dose range (80 to 120 mg per day) did not provide additional efficacy on average, compared to the low dose range (20 to 60 mg per day).
Adjunctive Therapy with Lithium or Valproate
The efficacy of lurasidone hydrochloride tablets, as an adjunctive therapy with lithium or valproate, was established in a 6-week, multicenter, randomized, double-blind, placebo-controlled study of adult patients (mean age of 41.7 years, range 18 to 72) who met DSM-IV-TR criteria for major depressive episodes associated with bipolar I disorder, with or without rapid cycling, and without psychotic features (N=340). Patients who remained symptomatic after treatment with lithium or valproate were randomized to flexibly dosed lurasidone hydrochloride tablets 20 to 120 mg/day or placebo.
The primary rating instrument used to assess depressive symptoms in this study was the MADRS. The primary endpoint was the change from baseline in MADRS score at Week 6. The key secondary instrument was the CGI-BP-S scale.
Lurasidone hydrochloride tablets were superior to placebo in reduction of MADRS and CGI-BP-S scores at Week 6, as an adjunctive therapy with lithium or valproate (Table 37).
Table 37: Primary Efficacy Results for Adult Studies in Depressive Episodes Associated with Bipolar I Disorder (MADRS Scores)
|
Study |
Treatment Group |
Primary Efficacy Measure: MADRS | ||
|
Mean Baseline Score (SD) |
LS Mean Change from Baseline (SE) |
Placebo-subtracted Differencea (95% CI) | ||
|
Monotherapy |
Lurasidone hydrochloride tablets (20-60 mg/day)* |
30.3 (5.0) |
-15.4 (0.8) |
-4.6 (-6.9, -2.3) |
|
Lurasidone hydrochloride tablets (80-120 mg/day)* |
30.6 (4.9) |
-15.4 (0.8) |
-4.6 (-6.9, -2.3) | |
|
Placebo |
30.5 (5.0) |
-10.7 (0.8) |
- | |
|
Adjunctive Therapy study |
Lurasidone hydrochloride tablets (20-120 mg/day)* + lithium or valproate |
30.6 (5.3) |
-17.1 (0.9) |
-3.6 (-6.0, -1.1) |
|
Placebo + lithium or valproate |
30.8 (4.8) |
-13.5 (0.9) |
- |
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: confidence interval, unadjusted for multiple comparisons.
a Difference (drug minus placebo) in least-squares mean change from baseline.
- Treatment group statistically significantly superior to placebo.
Pediatric Patients (10 to 17 years)
The efficacy of lurasidone hydrochloride tablets was established in a 6-week, multicenter, randomized, double-blind, placebo-controlled study of pediatric patients (10 to 17 years) who met DSM-5 criteria for a major depressive episode associated with bipolar I disorder, with or without rapid cycling, and without psychotic features (N=343). Patients were randomized to flexibly dosed lurasidone hydrochloride tablets 20 to 80 mg/day or placebo. At the end of the clinical study, most patients (67%) received 20 mg/day or 40 mg/day.
The primary rating scale used to assess depressive symptoms in this study was the Children’s Depression Rating Scale, Revised (CDRS-R) total score. The CDRS-R is a 17-item clinician- rated scale with total scores ranging from 17 to 113. The primary endpoint was the change from baseline in CDRS-R score at Week 6. The key secondary endpoint was the change from baseline in CGI-BP-S depression score.
Lurasidone hydrochloride tablets was superior to placebo in reduction of CDRS-R total score and CGI-BP-S depression score at Week 6. The primary efficacy results are provided in Table 38.
Table 38: Primary Efficacy Results for the Study in Depressive Episodes Associated with Bipolar I Disorder (CDRS-R Total Score) in Pediatric Patients (10 to 17 years)****
|
Treatment Group |
Primary Efficacy Measure: CDRS-R | ||
|
Mean Baseline Score |
LS Mean Change from |
Placebo-subtracted Differencea | |
|
Lurasidone hydrochloride tablets (20 to 80 mg/day)* |
59.2 (8.24) |
-21.0 (1.06) |
-5.7 (-8.4,-3.0) |
|
58.6 (8.26) |
-15.3 (1.08) |
-- |
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: confidence interval, unadjusted for multiple comparisons.
a Difference (drug minus placebo) in least-squares mean change from baseline.
- Treatment group statistically significantly superior to placebo.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Lurasidone hydrochloride tablets are white to off-white, round (20 mg or 40 mg), white to off-white, capsule shape (60 mg), light-green to green, oval (80 mg) or white to off-white, oval (120 mg) and identified with strength- specific two-sided debossing, “20” on one side and “L” on other side (20 mg), “40” on one side and “L” on other side (40 mg), “60” on one side and “L” on other side (60mg), “80” on one side and “L” on other side (80 mg) or "120” on one side and “L” on other side (120 mg). Tablets are supplied in the following strengths and package configurations (Table 39).
Table 39: Package Configuration for Lurasidone Hydrochloride Tablets
|
Tablet Strength |
Package Configuration |
NDC Code |
|
20 mg |
Bottles of 30 |
67877-638-30 |
|
Bottles of 90 |
67877-638-90 | |
|
Bottles of 500 |
67877-638-05 | |
|
Box of 100 (Hospital Unit Dose) |
67877-638-38 Carton | |
|
40 mg |
Bottles of 30 |
67877-639-30 |
|
Bottles of 90 |
67877-639-90 | |
|
Bottles of 500 |
67877-639-05 | |
|
Box of 100 (Hospital Unit Dose) |
67877-639-38 Carton | |
|
60 mg |
Bottles of 30 |
67877-640-30 |
|
Bottles of 90 |
67877-640-90 | |
|
Bottles of 500 |
67877-640-05 | |
|
Box of 100 (Hospital Unit Dose) |
67877-640-38 Carton | |
|
80 mg |
Bottles of 30 |
67877-641-30 |
|
Bottles of 90 |
67877-641-90 | |
|
Bottles of 500 |
67877-641-05 | |
|
Box of 100 (Hospital Unit Dose) |
67877-641-38 Carton | |
|
120 mg |
Bottles of 30 |
67877-642-30 |
|
Bottles of 90 |
67877-642-90 | |
|
Bottles of 500 |
67877-642-05 | |
|
Box of 100 (Hospital Unit Dose) |
67877-642-38 Carton |
Storage
Store lurasidone hydrochloride tablets at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature].
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.2 Depressive Episodes Associated with Bipolar I Disorder
Adults
The recommended starting dose of lurasidone hydrochloride tablets is 20 mg given once daily as monotherapy or as adjunctive therapy with lithium or valproate. Initial dose titration is not required. Lurasidone hydrochloride tablets has been shown to be effective in a dose range of 20 mg per day to 120 mg per day as monotherapy or as adjunctive therapy with lithium or valproate [see Clinical Studies (14.2)]. The maximum recommended dose, as monotherapy or as adjunctive therapy with lithium or valproate, is 120 mg per day. In the monotherapy study, the higher dose range (80 mg to 120 mg per day) did not provide additional efficacy, on average, compared to the lower dose range (20 to 60 mg per day) [see Clinical Studies (14.2)].
Pediatric Patients (10 – 17 years)
The recommended starting dose of lurasidone hydrochloride tablets is 20 mg given once daily as monotherapy. Initial dose titration is not required. The dose may be increased after one week based on clinical response. Lurasidone hydrochloride tablets has been shown to be effective in a dose range of 20 mg per day to 80 mg per day as monotherapy. At the end of the clinical study, most of the patients (67%) received 20 mg or 40 mg once daily [see Clinical Studies (14.2)]. The maximum recommended dose is 80 mg per day.
The efficacy of lurasidone hydrochloride tablets in the treatment of mania associated with bipolar disorder has not been established.
2.3 Administration Information
Lurasidone hydrochloride tablets should be taken with food (at least 350 calories). Administration with food substantially increases the absorption of lurasidone hydrochloride tablets. Administration with food increases the AUC approximately 2-fold and increases the Cmax approximately 3-fold. In the clinical studies, lurasidone hydrochloride tablets were administered with food [see Clinical Pharmacology (12.3)].
The effectiveness of lurasidone hydrochloride tablets for longer-term use, that is, for more than 6 weeks, has not been established in controlled studies. Therefore, the physician who elects to use lurasidone hydrochloride tablets for extended periods should periodically re-evaluate the long-term usefulness of the drug for the individual patient [see Dosage and Administration (2.2)].
2.4 Dose Modifications for Renal Impairment
Dose adjustment is recommended in moderate (creatinine clearance: 30 to <50 mL/min) and severe renal impairment (creatinine clearance <30 mL/min) patients. The recommended starting dose is 20 mg per day. The dose in these patients should not exceed 80 mg per day [see Use in Specific Populations (8.6)].
2.5 Dose Modifications for Hepatic Impairment
Dose adjustment is recommended in moderate (Child-Pugh Score = 7 to 9) and severe hepatic impairment (Child-Pugh Score = 10 to 15) patients. The recommended starting dose is 20 mg per day. The dose in moderate hepatic impairment patients should not exceed 80 mg per day and the dose in severe hepatic impairment patients should not exceed 40 per mg/day [see Use in Specific Populations (8.7)].
2.6 Dose Modifications Due to Drug Interactions of CYP3A4 Inhibitors and
CYP3A4 Inducers
Concomitant Use with CYP3A4 Inhibitors
Lurasidone hydrochloride tablets should not be used concomitantly with a strong CYP3A4 inhibitor (e.g., ketoconazole, clarithromycin, ritonavir, voriconazole, mibefradil, etc.) [see Contraindications (4)].
If lurasidone hydrochloride tablets are being prescribed and a moderate CYP3A4 inhibitor (e.g. diltiazem, atazanavir, erythromycin, fluconazole, verapamil etc.) is added to the therapy, the lurasidone hydrochloride tablets dose should be reduced to half of the original dose level. Similarly, if a moderate CYP3A4 inhibitor is being prescribed and lurasidone hydrochloride tablets are added to the therapy, the recommended starting dose of lurasidone hydrochloride tablets is 20 mg per day, and the maximum recommended dose of lurasidone hydrochloride tablets is 80 mg per day [see Contraindications (4), Drug Interactions (7.1)].
Grapefruit and grapefruit juice should be avoided in patients taking lurasidone hydrochloride tablets, since these may inhibit CYP3A4 and alter lurasidone hydrochloride tablets concentrations [see Drug Interactions (7.1)].
Concomitant Use with CYP3A4 Inducers
Lurasidone hydrochloride tablets should not be used concomitantly with a strong CYP3A4 inducer (e.g., rifampin, avasimibe, St. John’s wort, phenytoin, carbamazepine, etc.) [see Contraindications (4); Drug Interactions (7.1)]. If lurasidone hydrochloride tablets are used concomitantly with a moderate CYP3A4 inducer, it may be necessary to increase the lurasidone hydrochloride tablets dose after chronic treatment (7 days or more) with the CYP3A4 inducer.
Lurasidone hydrochloride tablets should be taken with food (at least 350 calories). Administration with food substantially increases the absorption of lurasidone hydrochloride tablets (2.3, 12.3).
|
Indication |
Starting Dose |
Recommended Dose |
|
Bipolar Depression |
20 mg per day |
20 mg to 120 mg per day |
|
Bipolar Depression – |
20 mg per day |
20 mg to 80 mg per day |
• Moderate and Severe Renal Impairment: Recommended starting dose is 20 mg per day, and the maximum recommended dose is 80 mg per day (2.4, 8.6).
• Moderate and Severe Hepatic Impairment: Recommended starting dose is 20 mg per day. The maximum recommended dose is 80 mg per day in moderate hepatic impairment and 40 mg per day in severe hepatic impairment (2.5, 8.7).
• Concomitant Use of a Moderate CYP3A4 inhibitor (e**.**g., diltiazem):
• Lurasidone hydrochloride tablets dose should be reduced to half of the original dose level.
Recommended starting dose is 20 mg per day. Maximum recommended dose is 80 mg per day (2.6,7.1).
• Concomitant Use of a Moderate CYP3A4 Inducer:
It may be necessary to increase the dose of lurasidone hydrochloride tablets (2.6,7.1).
