Temcongyi
3d4fe8ea-459d-062e-e063-6294a90a3b71
HUMAN OTC DRUG LABEL
Aug 26, 2025
Hubei Redrui Building Materials Co., Ltd.
DUNS: 617973213
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Salicylic Acid Liquid
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Drug Labeling Information
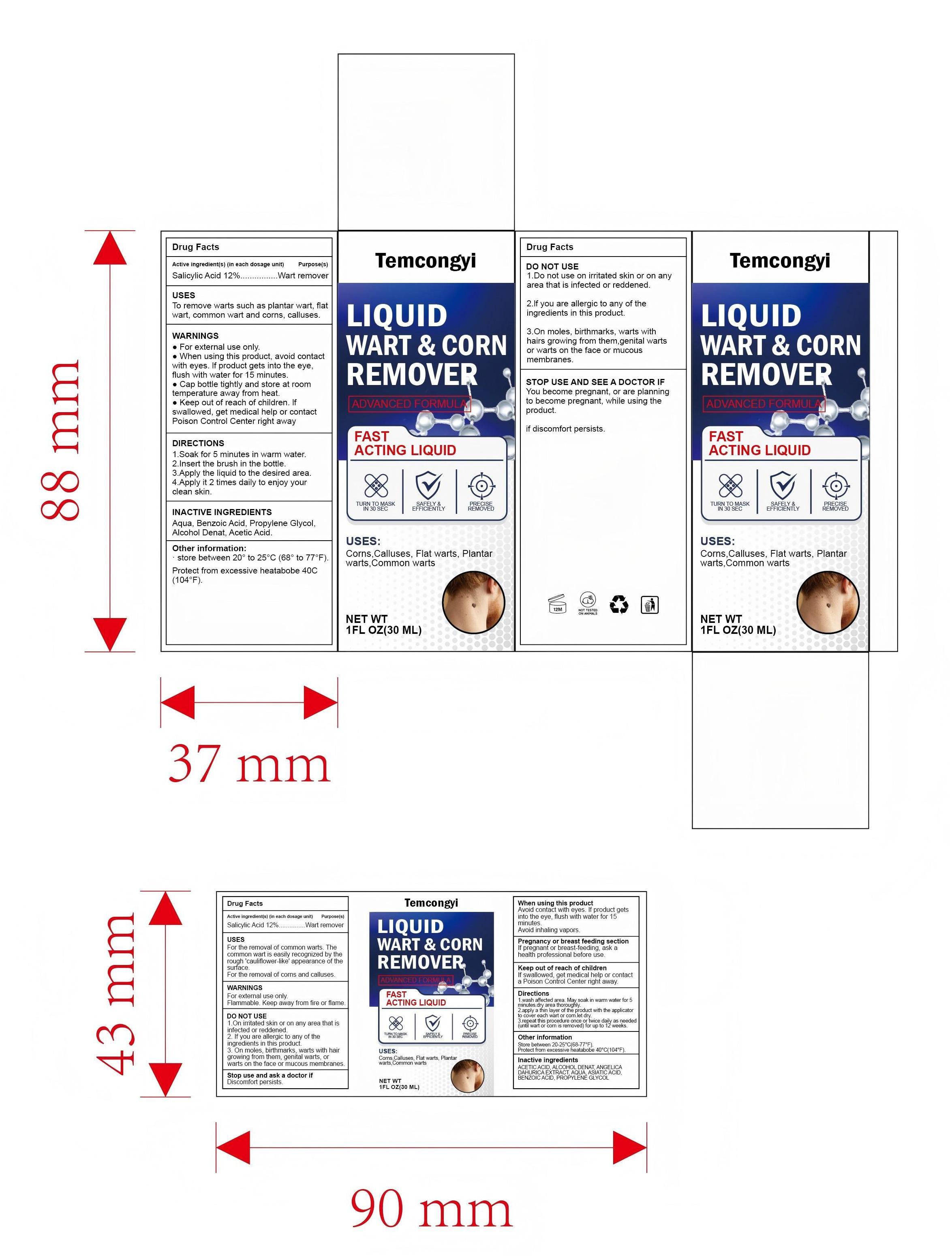
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Box Front Panel
ADVANCED FORMULA
FAST ACTING LIQUID
TURN TO MASK IN 30 SEC
SAFELY & EFFICIENTLY
PRECISE REMOVED
INDICATIONS & USAGE SECTION
Uses
- For the removal of common warts. The common wart is easily recognized by the rough 'cauliflower-like' appearance of the surface.
- For the removal of corns and calluses.
OTC - ACTIVE INGREDIENT SECTION
Active ingredient(s)
Salicylic acid 12%
OTC - PURPOSE SECTION
Purpose(s)
Wart and corn remover
WARNINGS SECTION
Warnings
- For external use only.
- Flammable. Keep away from fire or flame.
Do not use
- On irritated skin or on any area that is infected or reddened.
- If you are allergic to any of the ingredients in this product.
- On moles, birthmarks, warts with hair growing from them, genital warts, or warts on the face or mucous membranes.
When using this product
- Avoid contact with eyes. If product gets into the eye, flush with water for 15 minutes.
- Avoid inhaling vapors.
- Cap bottle tightly when not in use.
Stop use and ask a doctor if
- Discomfort persists.
OTC - PREGNANCY OR BREAST FEEDING SECTION
Pregnancy or breast feeding section
If pregnant or breast-feeding, ask a health professional before use.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
Directions
- Wash affected area.
- May soak wart in warm water for 5 minutes.
- Dry area thoroughly.
- Using the applicator, apply the liquid one drop at a time to sufficiently cover each wart, corn, or callus.
- Let dry.
- Repeat this procedure once or twice daily as needed (until wart, corn or callus is removed) for up to 12 weeks.
OTHER SAFETY INFORMATION
Other information
- Store between 20-25℃(68-77℉). Protect from excessive heatabobe 40℃(104℉).
INACTIVE INGREDIENT SECTION
Inactive ingredients
ACETIC ACID, ALCOHOL, ANGELICA DAHURICA ROOT, AQUA, ASIATIC ACID, BENZOIC ACID, PROPYLENE GLYCOL
OTC - QUESTIONS SECTION
Questions or comments?
934-935-7135 / Yiqinzhi726@gmail.com
