Ezetimibe and Simvastatin
These highlights do not include all the information needed to use EZETIMIBE AND SIMVASTATIN TABLETS safely and effectively. See full prescribing information for EZETIMIBE AND SIMVASTATIN TABLETS. EZETIMIBE and SIMVASTATIN tablets, for oral use Initial U.S. Approval: 2004
c90e8c96-e130-42d7-bf0d-5833652e40d5
HUMAN PRESCRIPTION DRUG LABEL
May 8, 2023
Ascend Laboratories, LLC
DUNS: 141250469
Products 4
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Ezetimibe and Simvastatin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (13)
Ezetimibe and Simvastatin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (13)
Ezetimibe and Simvastatin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (13)
Ezetimibe and Simvastatin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (13)
Drug Labeling Information
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Myopathy/Rhabdomyolysis
Simvastatin occasionally causes myopathy manifested as muscle pain, tenderness or weakness with creatine kinase above ten times the upper limit of normal (ULN). Myopathy sometimes takes the form of rhabdomyolysis with or without acute renal failure secondary to myoglobinuria, and rare fatalities have occurred. The risk of myopathy is increased by elevated plasma levels of simvastatin and simvastatin acid. Predisposing factors for myopathy include advanced age (≥65 years), female gender, uncontrolled hypothyroidism, and renal impairment. Chinese patients may be at increased risk for myopathy [see Use in Specific Populations (8.8)].
** The risk of myopathy, including rhabdomyolysis, is dose related.**In a clinical trial database in which 41,413 patients were treated with simvastatin, 24,747 (approximately 60%) of whom were enrolled in studies with a median follow-up of at least 4 years, the incidence of myopathy was approximately 0.03% and 0.08% at 20 and 40 mg/day, respectively. The incidence of myopathy with 80 mg (0.61%) was disproportionately higher than that observed at the lower doses. In these trials, patients were carefully monitored and some interacting medicinal products were excluded.
In a clinical trial in which 12,064 patients with a history of myocardial infarction were treated with simvastatin (mean follow-up 6.7 years), the incidence of myopathy (defined as unexplained muscle weakness or pain with a serum creatine kinase [CK] >10 times upper limit of normal [ULN]) in patients on 80 mg/day was approximately 0.9% compared with 0.02% for patients on 20 mg/day. The incidence of rhabdomyolysis (defined as myopathy with a CK >40 times ULN) in patients on 80 mg/day was approximately 0.4% compared with 0% for patients on 20 mg/day. The incidence of myopathy, including rhabdomyolysis, was highest during the first year and then notably decreased during the subsequent years of treatment. In this trial, patients were carefully monitored and some interacting medicinal products were excluded.
The risk of myopathy, including rhabdomyolysis, is greater in patients on simvastatin 80 mg compared with other statin therapies with similar or greater LDL-C-lowering efficacy and compared with lower doses of simvastatin. Therefore, the 10/80-mg dose ofEzetimibe and Simvastatin Tablets should be used only in patients who have been taking Ezetimibe and Simvastatin Tablets 10/80 mg chronically (e.g., for 12 months or more) without evidence of muscle toxicity[see Dosage and Administration, Restricted Dosing for 10/80 mg (2.2)].If, however, a patient who is currently tolerating the 10/80-mg dose of Ezetimibe and Simvastatin Tablets needs to be initiated on an interacting drug that is contraindicated or is associated with a dose cap for simvastatin, that patient should be switched to an alternative statin or statin-based regimen with less potential for the drug-drug interaction. Patients should be advised of the increased risk of myopathy, including rhabdomyolysis, and to report promptly any unexplained muscle pain, tenderness or weakness. If symptoms occur, treatment should be discontinued immediately[see Warnings and Precautions (5.2)].
In the Study of Heart and Renal Protection (SHARP), 9270 patients with chronic kidney disease were allocated to receive Ezetimibe and Simvastatin Tablets 10/20 mg daily (n=4650) or placebo (n=4620). During a median follow-up period of 4.9 years, the incidence of myopathy (defined as unexplained muscle weakness or pain with a serum creatine kinase [CK] >10 times upper limit of normal [ULN]) was 0.2% for Ezetimibe and Simvastatin Tablets and 0.1% for placebo: the incidence of rhabdomyolysis (defined as myopathy with a CK > 40 times ULN) was 0.09% for Ezetimibe and Simvastatin Tablets and 0.02% for placebo.
In postmarketing experience with ezetimibe, cases of myopathy and rhabdomyolysis have been reported. Most patients who developed rhabdomyolysis were taking a statin prior to initiating ezetimibe. However, rhabdomyolysis has been reported with ezetimibe monotherapy and with the addition of ezetimibe to agents known to be associated with increased risk of rhabdomyolysis, such as fibric acid derivatives. Ezetimibe and Simvastatin Tablets and a fenofibrate, if taking concomitantly, should both be immediately discontinued if myopathy is diagnosed or suspected.
**All patients starting therapy with****Ezetimibe and Simvastatin Tablets or whose dose of Ezetimibe and Simvastatin Tablets are being increased should be advised of the risk of myopathy, including rhabdomyolysis, and told to report promptly any unexplained muscle pain, tenderness or weakness particularly if accompanied by malaise or fever or if muscle signs and symptoms persist after discontinuing Ezetimibe and Simvastatin Tablets. Ezetimibe and Simvastatin Tablets therapy should be discontinued immediately if myopathy is diagnosed or suspected.**In most cases, muscle symptoms and CK increases resolved when simvastatin treatment was promptly discontinued. Periodic CK determinations may be considered in patients starting therapy with Ezetimibe and Simvastatin Tablets or whose dose is being increased, but there is no assurance that such monitoring will prevent myopathy.
Many of the patients who have developed rhabdomyolysis on therapy with simvastatin have had complicated medical histories, including renal insufficiency usually as a consequence of long-standing diabetes mellitus. Such patients taking Ezetimibe and Simvastatin Tablets merit closer monitoring.
Ezetimibe and Simvastatin Tablets therapy should be discontinued if markedly elevated CPK levels occur or myopathy is diagnosed or suspected. Ezetimibe and Simvastatin Tablets therapy should also be temporarily withheld in any patient experiencing an acute or serious condition predisposing to the development of renal failure secondary to rhabdomyolysis, e.g., sepsis; hypotension; major surgery; trauma; severe metabolic, endocrine, or electrolyte disorders; or uncontrolled epilepsy.
Drug Interactions
The risk of myopathy and rhabdomyolysis is increased by elevated plasma levels of simvastatin and simvastatin acid.Simvastatin is metabolized by the cytochrome P450 isoform 3A4. Certain drugs that inhibit this metabolic pathway can raise the plasma levels of simvastatin and may increase the risk of myopathy. These include itraconazole, ketoconazole, posaconazole, and voriconazole, the macrolide antibiotics erythromycin and clarithromycin, and the ketolide antibiotic telithromycin, HIV protease inhibitors, boceprevir, telaprevir, the antidepressant nefazodone, cobicistat-containing products, or grapefruit juice. [See Clinical Pharmacology (12.3).] Combination of these drugs with Ezetimibe and Simvastatin Tablets are contraindicated. If short- term treatment with strong CYP3A4 inhibitors is unavoidable, therapy with Ezetimibe and Simvastatin Tablets must be suspended during the course of treatment. [see Contraindications (4) and Drug Interactions (7).]
The combined use of Ezetimibe and Simvastatin Tablets with gemfibrozil, cyclosporine, or danazol is contraindicated [see Contraindications (4) and Drug Interactions (7.1 and 7.2)].
Caution should be used when prescribing fenofibrates with Ezetimibe and Simvastatin Tablets, as these agents can cause myopathy when given alone and the risk is increased when they are coadministered [see Drug Interactions (7.2, 7.7)].
Cases of myopathy, including rhabdomyolysis, have been reported with simvastatin coadministered with colchicine, and caution should be exercised when prescribing Ezetimibe and Simvastatin Tablets with colchicine [see Drug Interactions (7.9)].
The benefits of the combined use of Ezetimibe and Simvastatin Tablets with the following drugs should be carefully weighed against the potential risks of combinations: other lipid-lowering drugs (fenofibrates, or, for patients with HoFH, lomitapide), amiodarone, dronedarone, verapamil, diltiazem, amlodipine, or ranolazine [see Dosage and Administration (2.4),Drug Interactions (7.3)]
Cases of myopathy, including rhabdomyolysis, have been observed with simvastatin coadministered with lipid-modifying doses (≥1 g/day niacin) of niacin-containing products [see Drug Interactions (7.4)].
Cases of rhabdomyolysis have been reported with Ezetimibe and Simvastatin Tablets administered with daptomycin. Temporarily suspend Ezetimibe and Simvastatin Tablets in patients taking daptomycin [see Drug Interactions (7.10)]
Prescribing recommendations for interacting agents are summarized in Table 1 [see also Dosage and Administration (2.3, 2.4), Drug Interactions (7), and Clinical Pharmacology (12.3)].
Table 1: Drug Interactions Associated with Increased Risk of Myopathy/Rhabdomyolysis
|
Interacting Agents |
Prescribing Recommendations |
|
Strong CYP3A4 Inhibitors, e.g.: |
Contraindicated with Ezetimibe and Simvastatin Tablets |
|
Niacin (≥ 1g/day) |
For Chinese patients, not recommended with Ezetimibe and Simvastatin Tablets |
|
Verapamil |
Do not exceed 10/10 mg Ezetimibe and Simvastatin Tablets daily |
|
Amiodarone |
Do not exceed 10/20 mg Ezetimibe and Simvastatin Tablets daily |
|
Lomitapide |
For patients with HoFH, do not exceed 10/20 mg Ezetimibe and Simvastatin Tablets daily* |
|
Daptomycin |
Temporarily suspend ezetimibe and simvastatin tablets |
|
Grapefruit juice |
Avoid grapefruit juice |
- For patients with HoFH who have been taking 80 mg simvastatin chronically (e.g., for 12 months or more) without evidence of muscle toxicity, do not exceed 10/40 mg Ezetimibe and Simvastatin Tablets when taking lomitapide.
5.2 Immune-Mediated Necrotizing Myopathy
There have been rare reports of immune-mediated necrotizing myopathy (IMNM), an autoimmune myopathy, associated with statin use. IMNM is characterized by: proximal muscle weakness and elevated serum creatine kinase, which persist despite discontinuation of statin treatment; positive anti-HMG CoA reductase antibody; muscle biopsy showing necrotizing myopathy; and improvement with immunosuppressive agents. Additional neuromuscular and serologic testing may be necessary. Treatment with immunosuppressive agents may be required. Consider risk of IMNM carefully prior to initiation of a different statin. If therapy is initiated with a different statin, monitor for signs and symptoms of IMNM.
5.3 Liver Enzymes
In three placebo-controlled, 12-week trials, the incidence of consecutive elevations (≥3 X ULN) in serum transaminases was 1.7% overall for patients treated with Ezetimibe and Simvastatin Tablets and appeared to be dose-related with an incidence of 2.6% for patients treated with Ezetimibe and Simvastatin Tablets 10/80. In controlled long-term (48 week) extensions, which included both newly-treated and previously-treated patients, the incidence of consecutive elevations (≥3 X ULN) in serum transaminases was 1.8% overall and 3.6% for patients treated with Ezetimibe and Simvastatin Tablets 10/80. These elevations in transaminases were generally asymptomatic, not associated with cholestasis, and returned to baseline after discontinuation of therapy or with continued treatment.
In SHARP, 9270 patients with chronic kidney disease were allocated to receive Ezetimibe and Simvastatin Tablets 10/20 mg daily (n=4650), or placebo (n=4620). During a median follow-up period of 4.9 years, the incidence of consecutive elevations of transaminases (>3 X ULN) was 0.7% for Ezetimibe and Simvastatin Tablets and 0.6% for placebo.
It is recommended that liver function tests be performed before the initiation of treatment with Ezetimibe and Simvastatin Tablets, and thereafter when clinically indicated. There have been rare postmarketing reports of fatal and non-fatal hepatic failure in patients taking statins, including simvastatin. If serious liver injury with clinical symptoms and/or hyperbilirubinemia or jaundice occurs during treatment with Ezetimibe and Simvastatin Tablets, promptly interrupt therapy. If an alternate etiology is not found do not restart Ezetimibe and Simvastatin Tablets. Note that ALT may emanate from muscle, therefore ALT rising with CK may indicate myopathy [see Warnings and Precautions (5.1)].
Ezetimibe and Simvastatin Tablets should be used with caution in patients who consume substantial quantities of alcohol and/or have a past history of liver disease. Active liver diseases or unexplained persistent transaminase elevations are contraindications to the use of Ezetimibe and Simvastatin Tablets.
5.4 Endocrine Function
Increases in HbA1c and fasting serum glucose levels have been reported with HMG-CoA reductase inhibitors, including simvastatin.
·Patients should be advised of the increased risk of myopathy, including rhabdomyolysis, with the 10/80-mg dose.****(5.1)
· Patients should be advised to report promptly any unexplained and/or persistent muscle pain, tenderness, or weakness. Ezetimibe and Simvastatin Tablets should be discontinued immediately if myopathy is diagnosed or suspected. (5.1)
· Skeletal muscle effects (e.g., myopathy and rhabdomyolysis): Risks increase with higher doses and concomitant use of certain medicines. Predisposing factors include advanced age (≥65), female gender, uncontrolled hypothyroidism, and renal impairment. Rare cases of rhabdomyolysis with acute renal failure secondary to myoglobinuria have been reported. (4, 5.1,8.5, 8.6)
· Immune-Mediated Necrotizing Myopathy (IMNM): There have been rare reports of IMNM, an autoimmune myopathy, associated with statin use. IMNM is characterized by: proximal muscle weakness and elevated serum creatine kinase, which persist despite discontinuation of statin treatment; positive anti-HMG CoA reductase antibody; muscle biopsy showing necrotizing myopathy; and improvement with immunosuppressive agents. (5.2)
· Liver enzyme abnormalities: Persistent elevations in hepatic transaminases can occur. Check liver enzyme tests before initiating therapy and as clinically indicated thereafter. (5.3)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections of the label:
• Rhabdomyolysis and myopathy [see Warnings and Precautions (5.1)]
• Liver enzyme abnormalities [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Ezetimibe and Simvastatin Tablets********
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In the Ezetimibe and Simvastatin Tablets (ezetimibe/simvastatin) placebo- controlled clinical trials database of 1420 patients (age range 20 to 83 years, 52% women, 87% Caucasians, 3% Blacks, 5% Hispanics, 3% Asians) with a median treatment duration of 27 weeks, 5% of patients on Ezetimibe and Simvastatin Tablets and 2.2% of patients on placebo discontinued due to adverse reactions.
The most common adverse reactions in the group treated with Ezetimibe and Simvastatin Tablets that led to treatment discontinuation and occurred at a rate greater than placebo were:
· Increased ALT (0.9%)
· Myalgia (0.6%)
· Increased AST (0.4%)
· Back pain (0.4%)
The most commonly reported adverse reactions (incidence ≥2% and greater than placebo) in controlled clinical trials were: headache (5.8%), increased ALT (3.7%), myalgia (3.6%), upper respiratory tract infection (3.6%), and diarrhea (2.8%).
Ezetimibe and Simvastatin Tablets has been evaluated for safety in more than 10,189 patients in clinical trials.
Table 2 summarizes the frequency of clinical adverse reactions reported in ≥2% of patients treated with Ezetimibe and Simvastatin Tablets (n=1420) and at an incidence greater than placebo, regardless of causality assessment, from four placebo-controlled trials.
Table 2*******: Clinical Adverse Reactions Occurring in**≥2% of Patients Treated withEzetimibe and Simvastatin Tablets and at an Incidence Greater than Placebo, Regardless of Causality
|
Body System/Organ Class |
Placebo (%) n=371 |
Ezetimibe 10 mg (%) n=302 |
Simvastatin† (%) n=1234 |
Ezetimibe and Simvastatin† Tablets |
|
Body as a whole – general disorders | ||||
|
Headache |
5.4 |
6.0 |
5.9 |
5.8 |
|
Gastrointestinal system disorders | ||||
|
Diarrhea |
2.2 |
5.0 |
3.7 |
2.8 |
|
Infections and infestations | ||||
|
Influenza |
0.8 |
1.0 |
1.9 |
2.3 |
|
Upper respiratory tract infection |
2.7 |
5.0 |
5.0 |
3.6 |
|
Musculoskeletal and connective tissue disorders | ||||
|
Myalgia |
2.4 |
2.3 |
2.6 |
3.6 |
|
Pain in extremity |
1.3 |
3.0 |
2.0 |
2.3 |
*Includes two placebo-controlled combination studies in which the active ingredients equivalent to Ezetimibe and Simvastatin Tablets were coadministered and two placebo-controlled studies in which Ezetimibe and Simvastatin Tablet was administered.
†All doses.
Study of Heart and Renal Protection
In SHARP, 9270 patients were allocated to Ezetimibe and Simvastatin Tablets 10/20 mg daily (n=4650) or placebo (n=4620) for a median follow-up period of 4.9 years. The proportion of patients who permanently discontinued study treatment as a result of either an adverse event or abnormal safety blood result was 10.4% vs. 9.8% among patients allocated to Ezetimibe and Simvastatin Tablets and placebo, respectively. Comparing those allocated to Ezetimibe and Simvastatin Tablets vs. placebo, the incidence of myopathy (defined as unexplained muscle weakness or pain with a serum CK >10 times ULN) was 0.2% vs. 0.1% and the incidence of rhabdomyolysis (defined as myopathy with a CK >40 times ULN) was 0.09% vs. 0.02%, respectively. Consecutive elevations of transaminases (>3 X ULN) occurred in 0.7% vs. 0.6%, respectively. Patients were asked about the occurrence of unexplained muscle pain or weakness at each study visit: 21.5% vs. 20.9% patients ever reported muscle symptoms in the Ezetimibe and Simvastatin Tablets and placebo groups, respectively. Cancer was diagnosed during the trial in 9.4% vs. 9.5% of patients assigned to Ezetimibe and Simvastatin Tablets and placebo, respectively.
Ezetimibe
Other adverse reactions reported with ezetimibe in placebo-controlled studies, regardless of causality assessment: Musculoskeletal system disorders: arthralgia; Infections and infestations: sinusitis; Body as a whole – general disorders: fatigue.
Simvastatin
In a clinical trial in which 12,064 patients with a history of myocardial infarction were treated with simvastatin (mean follow-up 6.7 years), the incidence of myopathy (defined as unexplained muscle weakness or pain with a serum creatine kinase [CK] >10 times upper limit of normal [ULN]) in patients on 80 mg/day was approximately 0.9% compared with 0.02% for patients on 20 mg/day. The incidence of rhabdomyolysis (defined as myopathy with a CK >40 times ULN) in patients on 80 mg/day was approximately 0.4% compared with 0% for patients on 20 mg/day. The incidence of myopathy, including rhabdomyolysis, was highest during the first year and then notably decreased during the subsequent years of treatment. In this trial, patients were carefully monitored and some interacting medicinal products were excluded.
Other adverse reactions reported with simvastatin in placebo-controlled
clinical studies, regardless of causality assessment: Cardiac disorders:
atrial fibrillation; Ear and labyrinth disorders: vertigo; Gastrointestinal
disorders: abdominal pain, constipation, dyspepsia, flatulence, gastritis;
Skin and subcutaneous tissue disorders: eczema, rash; Endocrine disorders:
diabetes mellitus; Infections and infestations: bronchitis, sinusitis, urinary
tract infections; Body as a whole – general disorders: asthenia,
edema/swelling; Psychiatric disorders: insomnia.
Laboratory Tests
Marked persistent increases of hepatic serum transaminases have been noted [see Warnings and Precautions (5.3)]. Elevated alkaline phosphatase and γ-glutamyl transpeptidase have been reported. About 5% of patients taking simvastatin had elevations of CK levels of 3 or more times the normal value on one or more occasions. This was attributable to the noncardiac fraction of CK [see Warnings and Precautions (5.1)].
6.2 Postmarketing Experience
Because the below reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following adverse reactions have been reported in postmarketing experience for Ezetimibe and Simvastatin Tablets or ezetimibe or simvastatin: pruritus; alopecia; erythema multiforme; a variety of skin changes (e.g., nodules, discoloration, dryness of skin/mucous membranes, changes to hair/nails); dizziness; muscle cramps; myalgia; arthralgia; pancreatitis; paresthesia; peripheral neuropathy; vomiting; nausea; anemia; erectile dysfunction; interstitial lung disease; myopathy/rhabdomyolysis [see Warnings and Precautions (5.1)]; hepatitis/jaundice; fatal and non-fatal hepatic failure; depression; cholelithiasis; cholecystitis; thrombocytopenia; elevations in liver transaminases; elevated creatine phosphokinase.
There have been rare reports of immune-mediated necrotizing myopathy associated with statin use. [see Warnings and Precautions (5.1)].
Hypersensitivity reactions, including anaphylaxis, angioedema, rash, and urticaria have been reported.
In addition, an apparent hypersensitivity syndrome has been reported rarely that has included one or more of the following features: anaphylaxis, angioedema, lupus erythematous-like syndrome, polymyalgia rheumatica, dermatomyositis, vasculitis, purpura, thrombocytopenia, leukopenia, hemolytic anemia, positive ANA, ESR increase, eosinophilia, arthritis, arthralgia, urticaria, asthenia, photosensitivity, fever, chills, flushing, malaise, dyspnea, toxic epidermal necrolysis, erythema multiforme, including Stevens- Johnson syndrome.
There have been rare postmarketing reports of cognitive impairment (e.g., memory loss, forgetfulness, amnesia, memory impairment, confusion) associated with statin use. These cognitive issues have been reported for all statins. The reports are generally nonserious, and reversible upon statin discontinuation, with variable times to symptom onset (1 day to years) and symptom resolution (median of 3 weeks).
· Common (incidence ≥2% and greater than placebo) adverse reactions in clinical trials: headache, increased ALT, myalgia, upper respiratory tract infection, and diarrhea. (6.1)
To report SUSPECTED ADVERSE REACTIONS,contact Ascend Laboratories,LLC at 1-877-ASC-RX01 (877-272-7901) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
[See Clinical Pharmacology (12.3).]
Ezetimibe and Simvastatin Tablets
7.1 Strong CYP3A4 Inhibitors, Cyclosporine, or Danazol
Strong CYP3A4 inhibitors: The risk of myopathy is increased by reducing the elimination of the simvastatin component of Ezetimibe and Simvastatin Tablets. Hence when Ezetimibe and Simvastatin Tablets are used with an inhibitor of CYP3A4 (e.g., as listed below), elevated plasma levels of HMG-CoA reductase inhibitory activity increases the risk of myopathy and rhabdomyolysis, particularly with higher doses of Ezetimibe and Simvastatin Tablets. [See Warnings and Precautions (5.1) and Clinical Pharmacology (12.3).] Concomitant use of drugs labeled as having a strong inhibitory effect on CYP3A4 is contraindicated [see Contraindications (4)]. If treatment with itraconazole, ketoconazole, posaconazole, voriconazole, erythromycin, clarithromycin or telithromycin is unavoidable, therapy with Ezetimibe and Simvastatin Tablets must be suspended during the course of treatment.
Cyclosporine or Danazol: The risk of myopathy, including rhabdomyolysis is increased by concomitant administration of cyclosporine or danazol. Therefore, concomitant use of these drugs is contraindicated [see Contraindications (4), Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)].
7.2 Lipid-Lowering Drugs That Can Cause Myopathy When Given Alone
Gemfibrozil: Contraindicated with Ezetimibe and Simvastatin Tablets[see Contraindications(4) and Warnings and Precautions (5.1)].
Fenofibrates (e.g., fenofibrate and fenofibric acid): Caution should be used when prescribing with Ezetimibe and Simvastatin Tablets[see Warnings and Precautions(5.1) and Drug Interactions (7.7)].
7.3 Amiodarone, Dronedarone, Ranolazine, or Calcium Channel Blockers
The risk of myopathy, including rhabdomyolysis, is increased by concomitant administration of amiodarone, dronedarone, ranolazine, or calcium channel blockers such as verapamil, diltiazem or amlodipine [see Dosage and Administration (2.3) and Warnings and Precautions (5.1) and Table 6 in Clinical Pharmacology (12.3)].
7.4 Niacin
Cases of myopathy/rhabdomyolysis have been observed with simvastatin coadministered with lipid-modifying doses (≥1 g/day niacin) of niacin- containing products. The risk of myopathy is greater in Chinese patients. In a clinical trial (median follow-up 3.9 years) involving patients at high risk of cardiovascular disease and with well-controlled LDL-C levels on simvastatin 40 mg/day with or without ezetimibe 10 mg/day, there was no incremental benefit on cardiovascular outcomes with the addition of lipid-modifying doses (≥1 g/day) of niacin. Coadministration of Ezetimibe and Simvastatin Tablets with lipid-modifying doses (≥1g/day) of niacin is not recommended in Chinese patients. It is unknown if this risk applies to other Asian patients [see Warnings and Precautions (5.1) and Use in Specific Populations (8.8)].
7.5 Cholestyramine
Concomitant cholestyramine administration decreased the mean AUC of total ezetimibe approximately 55%. The incremental LDL-C reduction due to adding Ezetimibe and Simvastatin Tablets to cholestyramine may be reduced by this interaction.
7.6 Digoxin
In one study, concomitant administration of digoxin with simvastatin resulted in a slight elevation in plasma digoxin concentrations. Patients taking digoxin should be monitored appropriately when Ezetimibe and Simvastatin Tablets are initiated.
7.7 Fenofibrates (e.g., fenofibrate and fenofibric acid)
The safety and effectiveness of Ezetimibe and Simvastatin Tablets administered with fibrates have not been established. Because it is known that the risk of myopathy during treatment with HMG-CoA reductase inhibitors is increased with concurrent administration of fenofibrates, Ezetimibe and Simvastatin Tablets should be administered with caution when used concomitantly with a fenofibrate [see Warnings and Precautions (5.1)].
Fenofibrates may increase cholesterol excretion into the bile, leading to cholelithiasis. In a preclinical study in dogs, ezetimibe increased cholesterol in the gallbladder bile [see Animal Toxicology and/or Pharmacology (13.2)]. If cholelithiasis is suspected in a patient receiving Ezetimibe and Simvastatin Tablets and a fenofibrate, gallbladder studies are indicated and alternative lipid-lowering therapy should be considered [see the product labeling for fenofibrate and fenofibric acid].
7.8 Coumarin Anticoagulants
Simvastatin 20 to 40 mg/day modestly potentiated the effect of coumarin anticoagulants: the prothrombin time, reported as International Normalized Ratio (INR), increased from a baseline of 1.7 to 1.8 and from 2.6 to 3.4 in a normal volunteer study and in a hypercholesterolemic patient study, respectively. With other statins, clinically evident bleeding and/or increased prothrombin time has been reported in a few patients taking coumarin anticoagulants concomitantly. In such patients, prothrombin time should be determined before starting Ezetimibe and Simvastatin Tablets and frequently enough during early therapy to ensure that no significant alteration of prothrombin time occurs. Once a stable prothrombin time has been documented, prothrombin times can be monitored at the intervals usually recommended for patients on coumarin anticoagulants. If the dose of Ezetimibe and Simvastatin Tablets are changed or discontinued, the same procedure should be repeated. Simvastatin therapy has not been associated with bleeding or with changes in prothrombin time in patients not taking anticoagulants.
Concomitant administration of ezetimibe (10 mg once daily) had no significant effect on bioavailability of warfarin and prothrombin time in a study of twelve healthy adult males. There have been postmarketing reports of increased INR in patients who had ezetimibe added to warfarin. Most of these patients were also on other medications.
The effect of Ezetimibe and Simvastatin Tablets on the prothrombin time has not been studied.
7.9 Colchicine
Cases of myopathy, including rhabdomyolysis, have been reported with simvastatin coadministered with colchicine, and caution should be exercised when prescribing Ezetimibe and Simvastatin Tablets with colchicine.
7.10 Daptomycin
Cases of rhabdomyolysis have been reported with Ezetimibe and Simvastatin Tablets administered with daptomycin. Both Ezetimibe and Simvastatin Tablets and daptomycin can cause myopathy and rhabdomyolysis when given alone and the risk of myopathy and rhabdomyolysis may be increased by coadministration. Temporarily suspend Ezetimibe and Simvastatin Tablets in patients taking daptomycin [see Warnings and Precautions (5.1)].
Drug Interactions Associated with Increased****Risk of Myopathy/Rhabdomyolysis (2.3,2.4,4,5.1, 7.1,7.2,7.3,7.8,12.3)
|
Interacting Agents |
Prescribing****Recommendations |
|
Strong CYP3A4 Inhibitors, (e.g., itraconazole, ketoconazole, |
Contraindicated with Ezetimibe and Simvastatin Tablets |
|
Niacin (≥ 1g/day) |
For Chinese patients, not recommended with Ezetimibe and Simvastatin Tablets |
|
Verapamil, diltiazem, dronedarone |
Do not exceed 10/10 mg Ezetimibe and Simvastatin Tablets daily |
|
Amiodarone, amlodipine, ranolazine |
Do not exceed 10/20 mg Ezetimibe and Simvastatin Tablets daily |
|
Lomitapide |
For patients with HoFH, do not exceed 10/20 mg Ezetimibe and Simvastatin Tablets daily* |
|
Daptomycin |
Temporarily suspend ezetimibe and simvastatin tablets |
|
Grapefruit juice |
Avoid grapefruit juice |
-
For patients with HoFH who have been taking 80 mg simvastatin chronically (e.g., for 12 months or more) without evidence of muscle toxicity, do not exceed 10/40 mg Ezetimibe and Simvastatin Tablets when taking lomitapide.
- Coumarin anticoagulants: simvastatin prolongs INR. Achieve stable INR prior to starting Ezetimibe and Simvastatin Tablets. Monitor INR frequently until stable upon initiation or alteration of Ezetimibe and Simvastatin Tablets therapy. (7.8)
- Cholestyramine: Combination decreases exposure of ezetimibe. (2.3, 7.5)
- Other Lipid-lowering Medications: Use with fenofibrates increases the risk of adverse skeletal muscle effects. Caution should be used when prescribing with Ezetimibe and Simvastatin Tablets. (5.1, 7.2)
- Fenofibrates: Combination increases exposure of ezetimibe. If cholelithiasis is suspected in a patient receiving ezetimibe and a fenofibrate, gallbladder studies are indicated and alternative lipid- lowering therapy should be considered. (7.2, 7.7, 12.3)
DESCRIPTION SECTION
11 DESCRIPTION
Ezetimibe and Simvastatin Tablets contains ezetimibe, USP, a selective inhibitor of intestinal cholesterol and related phytosterol absorption, and simvastatin, USP, an HMG-CoA reductase inhibitor.
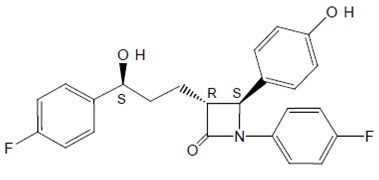
The chemical name of ezetimibe, USP is 1-(4-fluorophenyl)-3(R)-[3-(4-fluorophenyl)-3(S)-hydroxypropyl]-4(S)-(4-hydroxyphenyl)-2-azetidinone. The empirical formula is C24H21F2NO3 and its molecular weight is 409.43.
Ezetimibe, USP is a white to off white, crystalline powder, hygroscopic that
is soluble in methanol. Its structural formula is:
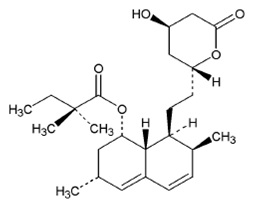
Simvastatin, USP, an inactive lactone, is hydrolyzed to the corresponding β-hydroxyacid form, which is an inhibitor of HMG-CoA reductase. Simvastatin, USP is butanoic acid, 2, 2-dimethyl-,1,2,3,7,8,8a-hexahydro-3,7dimethyl-8-[2-(tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl)-ethyl]-1-naphthalenyl ester, [1S-[1α,3α,7β,8β(2S*,4S*),-8aβ]]. The empirical formula of simvastatin, USP is C25H38O5 and its molecular weight is 418.57.
Simvastatin, USP is a white to off-white powder that is freely soluble in
chloroform, methanol and alcohol. Sparingly soluble in Propylene glycol, very
slightly soluble in Hexane. Practically insoluble in water. Its structural
formula is:
Ezetimibe and Simvastatin Tablets is available for oral use as tablets containing 10 mg of ezetimibe, USP, and 10 mg of simvastatin, USP (Ezetimibe and Simvastatin Tablets 10/10), 20 mg of simvastatin, USP (Ezetimibe and Simvastatin Tablets 10/20), 40 mg of simvastatin, USP (Ezetimibe and Simvastatin Tablets 10/40), or 80 mg of simvastatin, USP (Ezetimibe and Simvastatin Tablets 10/80). Each tablet contains the following inactive ingredients: Lactose Monohydrate, Microcrystalline Cellulose, Croscarmellose Sodium, Sodium Lauryl Sulfate, Hypromellose, Citric Acid Monohydrate, Propyl Gallate, Butylated Hydroxy Anisole and Magnesium Stearate.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Primary Hyperlipidemia
Ezetimibe and Simvastatin Tablets
Ezetimibe and Simvastatin Tablets reduces total-C, LDL-C, Apo B, TG, and non-
HDL-C, and increases HDL-C in patients with hyperlipidemia. Maximal to near
maximal response is generally achieved within 2 weeks and maintained during
chronic therapy.
Ezetimibe and Simvastatin Tablets are effective in men and women with
hyperlipidemia. Experience in non-Caucasians is limited and does not permit a
precise estimate of the magnitude of the effects of Ezetimibe and Simvastatin
Tablets.
Five multicenter, double-blind studies conducted with either Ezetimibe and
Simvastatin Tablets or coadministered ezetimibe and simvastatin equivalent to
Ezetimibe and Simvastatin Tablets in patients with primary hyperlipidemia are
reported: two were comparisons with simvastatin, two were comparisons with
atorvastatin, and one was a comparison with rosuvastatin.
In a multicenter, double-blind, placebo-controlled, 12-week trial, 1528
hyperlipidemic patients were randomized to one of ten treatment groups:
placebo, ezetimibe (10 mg), simvastatin (10 mg, 20 mg, 40 mg, or 80 mg), or
Ezetimibe and Simvastatin Tablets (10/10, 10/20, 10/40, or 10/80).
When patients receiving Ezetimibe and Simvastatin Tablets were compared to
those receiving all doses of simvastatin, Ezetimibe and Simvastatin Tablets
significantly lowered total-C, LDL-C, Apo B, TG, and non-HDL-C. The effects of
Ezetimibe and Simvastatin Tablets on HDL-C were similar to the effects seen
with simvastatin. Further analysis showed Ezetimibe and Simvastatin Tablets
significantly increased HDL-C compared with placebo. (See Table 7.) The lipid
response to Ezetimibe and Simvastatin Tablets was similar in patients with TG
levels greater than or less than 200 mg/dL.
Table 7: Response to****Ezetimibe and Simvastatin Tablets in Patients with Primary Hyperlipidemia
(Mean* % Change from Untreated Baseline†)
|
Treatment |
N |
Total-C |
LDL-C |
Apo B |
HDL-C |
TG* |
Non-HDL- |
|
Pooled data (All Ezetimibe and Simvastatin Tablets doses)‡ |
609 |
-38 |
-53 |
-42 |
+7 |
-24 |
-49 |
|
Pooled data (All simvastatin |
622 |
-28 |
-39 |
-32 |
+7 |
-21 |
-36 |
|
Ezetimibe 10 mg |
149 |
-13 |
-19 |
-15 |
+5 |
-11 |
-18 |
|
Placebo |
148 |
-1 |
-2 |
0 |
0 |
-2 |
-2 |
|
Ezetimibe and Simvastatin Tablets by dose | |||||||
|
10-10 |
152 |
-31 |
-45 |
-35 |
+8 |
-23 |
-41 |
|
10-20 |
156 |
-36 |
-52 |
-41 |
+10 |
-24 |
-47 |
|
10-40 |
147 |
-39 |
-55 |
-44 |
+6 |
-23 |
-51 |
|
10-80 |
154 |
-43 |
-60 |
-49 |
+6 |
-31 |
-56 |
|
Simvastatin by dose | |||||||
|
10 mg |
158 |
-23 |
-33 |
-26 |
+5 |
-17 |
-30 |
|
20 mg |
150 |
-24 |
-34 |
-28 |
+7 |
-18 |
-32 |
|
40 mg |
156 |
-29 |
-41 |
-33 |
+8 |
-21 |
-38 |
|
80 mg |
158 |
-35 |
-49 |
-39 |
+7 |
-27 |
-45 |
- For triglycerides, median % change from baseline.
† Baseline - on no lipid-lowering drug.
‡ Ezetimibe and Simvastatin Tablets doses pooled (10/10 to 10/80) significantly reduced total-C, LDL-C, Apo B, TG, and non-HDL-C compared to simvastatin and significantly increased HDL-C compared to placebo.
In a multicenter, double-blind, controlled, 23-week study, 710 patients with
known CHD or CHD risk equivalents, as defined by the NCEP ATP III guidelines,
and an LDL-C ≥130 mg/dL were randomized to one of four treatment groups:
coadministered ezetimibe and simvastatin equivalent to Ezetimibe and
Simvastatin Tablets (10/10, 10/20, and 10/40) or simvastatin 20 mg. Patients
not reaching an LDL-C <100 mg/dL had their simvastatin dose titrated at 6-week
intervals to a maximal dose of 80 mg.
At Week 5, the LDL-C reductions with Ezetimibe and Simvastatin Tablets 10/10,
10/20, or 10/40 were significantly larger than with simvastatin 20 mg (see
Table 8).
** Table 8: Response to Ezetimibe and Simvastatin Tablets after 5 Weeks in Patients with CHD or CHD Risk Equivalents and an LDL-C≥130 mg/dL**
|
Simvastatin |
Ezetimibe and Simvastatin Tablets 10/10 |
Ezetimibe and Simvastatin Tablets 10/20 |
Ezetimibe and Simvastatin Tablets 10/40 | |
|
N |
253 |
251 |
109 |
97 |
|
Mean baseline LDL-C |
174 |
165 |
167 |
171 |
|
Percent change LDL-C |
-38 |
-47 |
-53 |
-59 |
In a multicenter, double-blind, 6-week study, 1902 patients with primary
hyperlipidemia, who had not met their NCEP ATP III target LDL-C goal, were
randomized to one of eight treatment groups: Ezetimibe and Simvastatin Tablets
(10/10, 10/20, 10/40, or 10/80) or atorvastatin (10 mg, 20 mg, 40 mg, or 80
mg).Across the dosage range, when patients receiving Ezetimibe and Simvastatin
Tablets were compared to those receiving milligram-equivalent statin doses of
atorvastatin, Ezetimibe and Simvastatin Tablets lowered total-C, LDL-C, Apo B,
and non- HDL-C significantly more than atorvastatin. Only the 10/40 mg and
10/80 mg Ezetimibe and Simvastatin Tablets doses increased HDL-C significantly
more than the corresponding milligram-equivalent statin dose of atorvastatin.
The effects of Ezetimibe and Simvastatin Tablets on TG were similar to the
effects seen with atorvastatin. (See Table 9.)
Table 9: Response to Ezetimibe and Simvastatin Tablets and Atorvastatin in
Patients with Primary Hyperlipidemia(Mean % Change from Untreated
Baseline***†****)**
|
Treatment | |||||||
|
(Daily Dose) |
N |
Total-C ‡ |
LDL-C‡ |
Apo B‡ |
HDL-C |
TG* |
Non-HDL-C‡ |
|
Ezetimibe and Simvastatin Tablets by dose | |||||||
|
10-10 |
230 |
-34§ |
-47§ |
-37§ |
+8 |
-26 |
-43§ |
|
10-20 |
233 |
-37§ |
-51§ |
-40§ |
+7 |
-25 |
-46§ |
|
10-40 |
236 |
-41§ |
-57§ |
-46§ |
+9§ |
-27 |
-52§ |
|
10-80 |
224 |
-43§ |
-59§ |
-48§ |
+8§ |
-31 |
-54§ |
|
Atorvastatin by dose | |||||||
|
10 mg |
235 |
-27 |
-36 |
-31 |
+7 |
-21 |
-34 |
|
20 mg |
230 |
-32 |
-44 |
-37 |
+5 |
-25 |
-41 |
|
40 mg |
232 |
-36 |
-48 |
-40 |
+4 |
-24 |
-45 |
|
80 mg |
230 |
-40 |
-53 |
-44 |
+1 |
-32 |
-50 |
- For triglycerides, median % change from baseline.
† Baseline - on no lipid-lowering drug.
‡ Ezetimibe and Simvastatin Tablets doses pooled (10/10 to 10/80) provided significantly greater reductions in total-C, LDL C, Apo B, and non-HDL-C compared to atorvastatin doses pooled (10 to 80).
§ p<0.05 for difference with atorvastatin at equal mg doses of the simvastatin component.
In a multicenter, double-blind, 24-week, forced-titration study, 788 patients with primary hyperlipidemia, who had not met their NCEP ATP III target LDL-C goal, were randomized to receive coadministered ezetimibe and simvastatin equivalent to Ezetimibe and Simvastatin Tablets (10/10 and 10/20) or atorvastatin 10 mg. For all three treatment groups, the dose of the statin was titrated at 6-week intervals to 80 mg. At each pre-specified dose comparison, Ezetimibe and Simvastatin Tablets lowered LDL-C to a greater degree than atorvastatin (see Table 10).
Table 10: Response toEzetimibe and Simvastatin Tablets and Atorvastatin in Patients with Primary Hyperlipidemia (Mean % Change from Untreated Baseline***†****)**
|
Treatment | |||||||
|
Week 6 |
N |
Total-C |
LDL-C |
Apo B |
HDL-C |
TG* |
Non-HDL-C |
|
Atorvastatin 10 mg‡ |
262 |
-28 |
-37 |
-32 |
+5 |
-23 |
-35 |
|
Ezetimibe and Simvastatin Tablets 10/10§ |
263 |
-34¶ |
-46¶ |
-38¶ |
+8¶ |
-26 |
-43¶ |
|
Ezetimibe and Simvastatin Tablets 10/20# |
263 |
-36¶ |
-50¶ |
-41¶ |
+10¶ |
-25 |
-46¶ |
|
Week 12 | |||||||
|
Atorvastatin 20 mg |
246 |
-33 |
-44 |
-38 |
+7 |
-28 |
-42 |
|
Ezetimibe and Simvastatin Tablets 10/20 |
250 |
-37¶ |
-50¶ |
-41¶ |
+9 |
-28 |
-46¶ |
|
Ezetimibe and Simvastatin Tablets 10/40 |
252 |
-39¶ |
-54¶ |
-45¶ |
+12¶ |
-31 |
-50¶ |
|
Week 18 | |||||||
|
Atorvastatin 40 mg |
237 |
-37 |
-49 |
-42 |
+8 |
-31 |
-47 |
|
Ezetimibe and Simvastatin Tablets 10/40Þ |
482 |
-40¶ |
-56¶ |
-45¶ |
+11¶ |
-32 |
-52¶ |
|
Week 24 | |||||||
|
Atorvastatin 80 mg |
228 |
-40 |
-53 |
-45 |
+6 |
-35 |
-50 |
|
Ezetimibe and Simvastatin Tablets 10/80Þ |
459 |
-43¶ |
-59¶ |
-49¶ |
+12¶ |
-35 |
-55¶ |
- For triglycerides, median % change from baseline.
† Baseline - on no lipid-lowering drug.
‡ Atorvastatin: 10 mg start dose titrated to 20 mg, 40 mg, and 80 mg through Weeks 6, 12, 18, and 24.
§ Ezetimibe and Simvastatin Tablets: 10/10 start dose titrated to 10/20, 10/40, and 10/80 through Weeks 6, 12, 18, and 24.
¶ p≤0.05 for difference with atorvastatin in the specified week.
Ezetimibe and Simvastatin Tablets: 10/20 start dose titrated to 10/40,
10/40, and 10/80 through Weeks 6, 12, 18, and 24.
ÞData pooled for common doses of Ezetimibe and Simvastatin Tablets at Weeks 18
and 24.
In a multicenter, double-blind, 6-week study, 2959 patients with primary
hyperlipidemia, who had not met their NCEP ATP III target LDL-C goal, were
randomized to one of six treatment groups: Ezetimibe and Simvastatin Tablets
(10/20, 10/40, or 10/80) or rosuvastatin (10 mg, 20 mg, or 40 mg).
The effects of Ezetimibe and Simvastatin Tablets and rosuvastatin on total-C,
LDL-C, Apo B, TG, non-HDL-C and HDL-C are shown in Table 11.
Table 11: Response to Ezetimibe and Simvastatin Tablets and Rosuvastatin in
Patients with Primary Hyperlipidemia (Mean % Change from Untreated
Baseline†)*
|
Treatment | |||||||
|
(Daily Dose) |
N |
Total-C ‡ |
LDL-C‡ |
Apo B‡ |
HDL-C |
TG* |
Non-HDL-C‡ |
|
Ezetimibe and Simvastatin Tablets by dose | |||||||
|
10-20 |
476 |
-37§ |
-52§ |
-42§ |
+7 |
-23§ |
-47§ |
|
10-40 |
477 |
-39¶ |
-55¶ |
-44¶ |
+8 |
-27 |
-50¶ |
|
10-80 |
474 |
-44# |
-61# |
-50# |
+8 |
-30# |
-56# |
|
Rosuvastatin by dose | |||||||
|
10 mg |
475 |
-32 |
-46 |
-37 |
+7 |
-20 |
-42 |
|
20 mg |
478 |
-37 |
-52 |
-43 |
+8 |
-26 |
-48 |
|
40 mg |
475 |
-41 |
-57 |
-47 |
+8 |
-28 |
-52 |
*For triglycerides, median % change from baseline.
† Baseline - on no lipid-lowering drug.
‡ Ezetimibe and Simvastatin Tablets doses pooled (10/20 to10/80) provided
significantly greater reductions in total-C, LDL-C, Apo B, and non-HDL-C
compared to rosuvastatin
doses pooled (10 to 40 mg).
§ p<0.05 vs. rosuvastatin 10 mg.
¶ p<0.05 vs. rosuvastatin 20 mg.
p<0.05 vs. rosuvastatin 40 mg.
In a multicenter, double-blind, 24-week trial, 214 patients with type 2
diabetes mellitus treated with thiazolidinediones (rosiglitazone or
pioglitazone) for a minimum of 3 months and simvastatin 20 mg for a minimum of
6 weeks were randomized to receive either simvastatin 40 mg or the
coadministered active ingredients equivalent to Ezetimibe and Simvastatin
Tablets 10/20. The median LDL-C and HbA1c levels at baseline were 89 mg/dL and
7.1%, respectively.
Ezetimibe and Simvastatin Tablets 10/20 was significantly more effective than
doubling the dose of simvastatin to 40 mg. The median percent changes from
baseline for Ezetimibe and Simvastatin Tablets vs. simvastatin were: LDL-C
-25% and -5%; total-C-16% and -5%; Apo B -19% and -5%; and non-HDL-C -23% and
-5%. Results for HDL-C and TG between the two treatment groups were not
significantly different.
Ezetimibe
In two multicenter, double-blind, placebo-controlled, 12-week studies in 1719
patients with primary hyperlipidemia, ezetimibe significantly lowered total-C
(-13%), LDL-C (-19%), Apo B (-14%), and TG (-8%), and increased HDL-C (+3%)
compared to placebo. Reduction in LDL-C was consistent across age, sex, and
baseline LDL-C.
Simvastatin
In two large, placebo-controlled clinical trials, the Scandinavian Simvastatin
Survival Study (N=4,444 patients) and the Heart Protection Study (N=20,536
patients), the effects of treatment with simvastatin were assessed in patients
at high risk of coronary events because of existing coronary heart disease,
diabetes, peripheral vessel disease, history of stroke or other
cerebrovascular disease. Simvastatin was proven to reduce: the risk of total
mortality by reducing CHD deaths; the risk of non-fatal myocardial infarction
and stroke; and the need for coronary and non-coronary revascularization
procedures.
No incremental benefit of Ezetimibe and Simvastatin Tablets on cardiovascular
morbidity and mortality over and above that demonstrated for simvastatin has
been established.
14.2 Homozygous Familial Hypercholesterolemia (HoFH)
A double-blind, randomized, 12-week study was performed in patients with a clinical and/or genotypic diagnosis of HoFH. Data were analyzed from a subgroup of patients (n=14) receiving simvastatin 40 mg at baseline. Increasing the dose of simvastatin from 40 to 80 mg (n=5) produced a reduction of LDL-C of 13% from baseline on simvastatin 40 mg. Coadministered ezetimibe and simvastatin equivalent to Ezetimibe and Simvastatin Tablets (10/40 and 10/80 pooled, n=9), produced a reduction of LDL-C of 23% from baseline on simvastatin 40 mg. In those patients coadministered ezetimibe and simvastatin equivalent to Ezetimibe and Simvastatin Tablets (10/80, n=5), a reduction of LDL-C of 29% from baseline on simvastatin 40 mg was produced.
14.3 Chronic Kidney Disease (CKD)
The Study of Heart and Renal Protection (SHARP) was a multinational,
randomized, placebo- controlled, double-blind trial that investigated the
effect of Ezetimibe and Simvastatin Tablets on the time to a first major
vascular event (MVE) among 9438 patients with moderate to severe chronic
kidney disease (approximately one- third on dialysis at baseline) who did not
have a history of myocardial infarction or coronary revascularization. An MVE
was defined as nonfatal MI, cardiac death, stroke, or any revascularization
procedure. Patients were allocated to treatment using a method that took into
account the distribution of 8 important baseline characteristics of patients
already enrolled and minimized the imbalance of those characteristics across
the groups.
For the first year, 9438 patients were allocated 4:4:1, to Ezetimibe and
Simvastatin Tablets 10/20, placebo, or simvastatin 20 mg daily, respectively.
The 1-year simvastatin arm enabled the comparison of Ezetimibe and Simvastatin
Tablets to simvastatin with regard to safety and effect on lipid levels. At 1
year the simvastatin-only arm was re-allocated 1:1 to Ezetimibe and
Simvastatin Tablets 10/20 or placebo. A total of 9270 patients were ever
allocated to Ezetimibe and Simvastatin Tablets 10/20 (n=4650) or placebo
(n=4620) during the trial. The median follow-up duration was 4.9 years.
Patients had a mean age of 61 years; 63% were male, 72% were Caucasian, and
23% were diabetic; and, for those not on dialysis at baseline, the median
serum creatinine was 2.5 mg/dL and the median estimated glomerular filtration
rate (eGFR) was 25.6 mL/min/1.73 m2, with 94% of patients having an eGFR < 45
mL/min/1.73 m2. Eligibility did not depend on lipid levels. Mean LDL-C at
baseline was 108 mg/dL. At 1 year, the mean LDL-C was 26% lower in the
simvastatin arm and 38% lower in the Ezetimibe and Simvastatin Tablets arm
relative to placebo. At the midpoint of the study (2.5 years), the mean LDL-C
was 32% lower for Ezetimibe and Simvastatin Tablets relative to placebo.
Patients no longer taking study medication were included in all lipid
measurements.
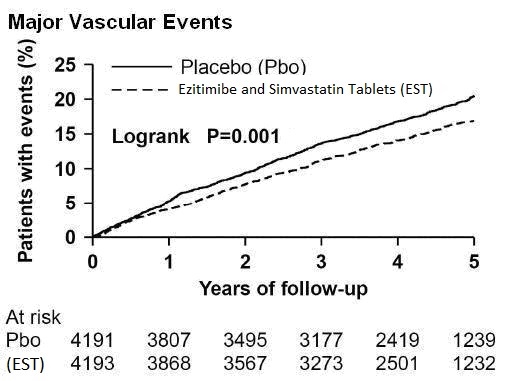
In the primary intent-to-treat analysis, 639 (15.2%) of 4193 patients
initially allocated to Ezetimibe and Simvastatin Tablets and 749 (17.9%) of
4191 patients initially allocated to placebo experienced an MVE. This
corresponded to a relative risk reduction of 16% (p=0.001) (see Figure 1).
Similarly, 526 (11.3%) of 4650 patients ever allocated to Ezetimibe and
Simvastatin Tablets and 619 (13.4%) of 4620 patients ever allocated to placebo
experienced a major atherosclerotic event (MAE; a subset of the MVE composite
that excluded non-coronary cardiac deaths and hemorrhagic stroke),
corresponding to a relative risk reduction of 17% (p=0.002). The trial
demonstrated that treatment with Ezetimibe and Simvastatin Tablets 10/20 mg
versus placebo reduced the risk for MVE and MAE in this CKD population. The
study design precluded drawing conclusions regarding the independent
contribution of either ezetimibe or simvastatin to the observed effect.
The treatment effect of Ezetimibe and Simvastatin Tablets on MVE was
attenuated among patients on dialysis at baseline compared with those not on
dialysis at baseline. Among 3023 patients on dialysis at baseline, Ezetimibe
and Simvastatin Tablets reduced the risk of MVE by 6% (RR 0.94: 95% CI 0.80 to
1.09) compared with 22% (RR 0.78: 95% CI 0.69 to 0.89) among 6247 patients not
on dialysis at baseline (interaction P=0.08).
Figure 1: Effect of Ezetimibe and Simvastatin Tablets on the Primary
Endpoint of Risk of Major Vascular Events
****
****The individual components of MVE in all patients ever allocated to
Ezetimibe and Simvastatin Tablets or placebo are presented in Table 12.
Table 12: Number of First Events for Each Component of the Major Vascular Event Composite Endpoint in SHARP*
|
Outcome |
Ezetimibe and Simvastatin Tablets 10/20 |
Placebo |
Risk Ratio |
P-value |
|
Major Vascular Events |
701 (15.1%) |
814 (17.6%) |
0.85 (0.77 to 0.94) |
0.001 |
|
Nonfatal MI |
134 (2.9%) |
159 (3.4%) |
0.84 (0.66 to 1.05) |
0.12 |
|
Cardiac Death |
253 (5.4%) |
272 (5.9%) |
0.93 (0.78 to 1.10) |
0.38 |
|
Any Stroke |
171 (3.7%) |
210 (4.5%) |
0.81 (0.66 to 0.99) |
0.038 |
|
Non-hemorrhagic Stroke |
131 (2.8%) |
174 (3.8%) |
0.75 (0.60 to 0.94) |
0.011 |
|
Hemorrhagic Stroke |
45 (1.0%) |
37 (0.8%) |
1.21 (0.78 to 1.86) |
0.40 |
|
Any Revascularization |
284 (6.1%) |
352 (7.6%) |
0.79 (0.68 to 0.93) |
0.004 |
*Intention-to-treat analysis on all SHARP patients ever allocated to Ezetimibe and Simvastatin Tablets or placebo.
Among patients not on dialysis at baseline, Ezetimibe and Simvastatin Tablets
did not reduce the risk of progressing to end- stage renal disease compared
with placebo (RR 0.97: 95% CI 0.89 to 1.05).
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Patients should be advised to adhere to their National Cholesterol Education
Program (NCEP) recommended diet, a regular exercise program, and periodic
testing of a fasting lipid panel.
Patients should be advised about substances they should not take
concomitantly with Ezetimibe and Simvastatin Tablets [see Contraindications (4) and Warnings and Precautions (5.1)].
Patients should also be advised to inform other healthcare professionals
prescribing a new medication or increasing the dose of an existing medication
that they are taking Ezetimibe and Simvastatin Tablets.
17.1 Muscle Pain
All patients starting therapy with Ezetimibe and Simvastatin Tablets should be advised of the risk of myopathy, including rhabdomyolysis, and told to report promptly any unexplained muscle pain, tenderness or weakness particularly if accompanied by malaise or fever or if these muscle signs or symptoms persist after discontinuing Ezetimibe and Simvastatin Tablets.Patients using the 10/80-mg dose should be informed that the risk of myopathy, including rhabdomyolysis, is increased with the use of the 10/80-mg dose. The risk of myopathy, including rhabdomyolysis, occurring with use of Ezetimibe and Simvastatin Tablets is increased when taking certain types of medication or consuming grapefruit juice. Patients should discuss all medication, both prescription and over the counter, with their healthcare professional.
17.2 Liver Enzymes
It is recommended that liver function tests be performed before the initiation of Ezetimibe and Simvastatin Tablets, and thereafter when clinically indicated. All patients treated with Ezetimibe and Simvastatin Tablets should be advised to report promptly any symptoms that may indicate liver injury, including fatigue, anorexia, right upper abdominal discomfort, dark urine or jaundice.
17.3 Pregnancy
Women of childbearing age should be advised to use an effective method of birth control to prevent pregnancy while using Ezetimibe and Simvastatin Tablets. Discuss future pregnancy plans with your patients, and discuss when to stop taking Ezetimibe and Simvastatin Tablets if they are trying to conceive. Patients should be advised that if they become pregnant they should stop taking Ezetimibe and Simvastatin Tablets and call their healthcare professional.
17.4 Breastfeeding
Women who are breastfeeding should be advised to not use Ezetimibe and Simvastatin Tablets. Patients who have a lipid disorder and are breastfeeding should be advised to discuss the options with their healthcare professional.
For Patient Information, please visit:
http://www.ascendlaboratories.com/pti/ezetim_simvatablets.pdf
Manufactured by:
Alkem Laboratories Ltd.,
INDIA.
Distributed by:
Ascend Laboratories, LLC
Parsippany, NJ 07054
Revised: December, 2022
PT2626-06
