Fosaprepitant
These highlights do not include all the information needed to use FOSAPREPITANT FOR INJECTION safely and effectively. See full prescribing information for FOSAPREPITANT FOR INJECTION. FOSAPREPITANT for injection, for intravenous use Initial U.S. Approval: 2008
2d30d839-bb88-46ce-a9ed-66fe32a12a7a
HUMAN PRESCRIPTION DRUG LABEL
Jul 7, 2025
BluePoint Laboratories
DUNS: 985523874
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Fosaprepitant dimeglumine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
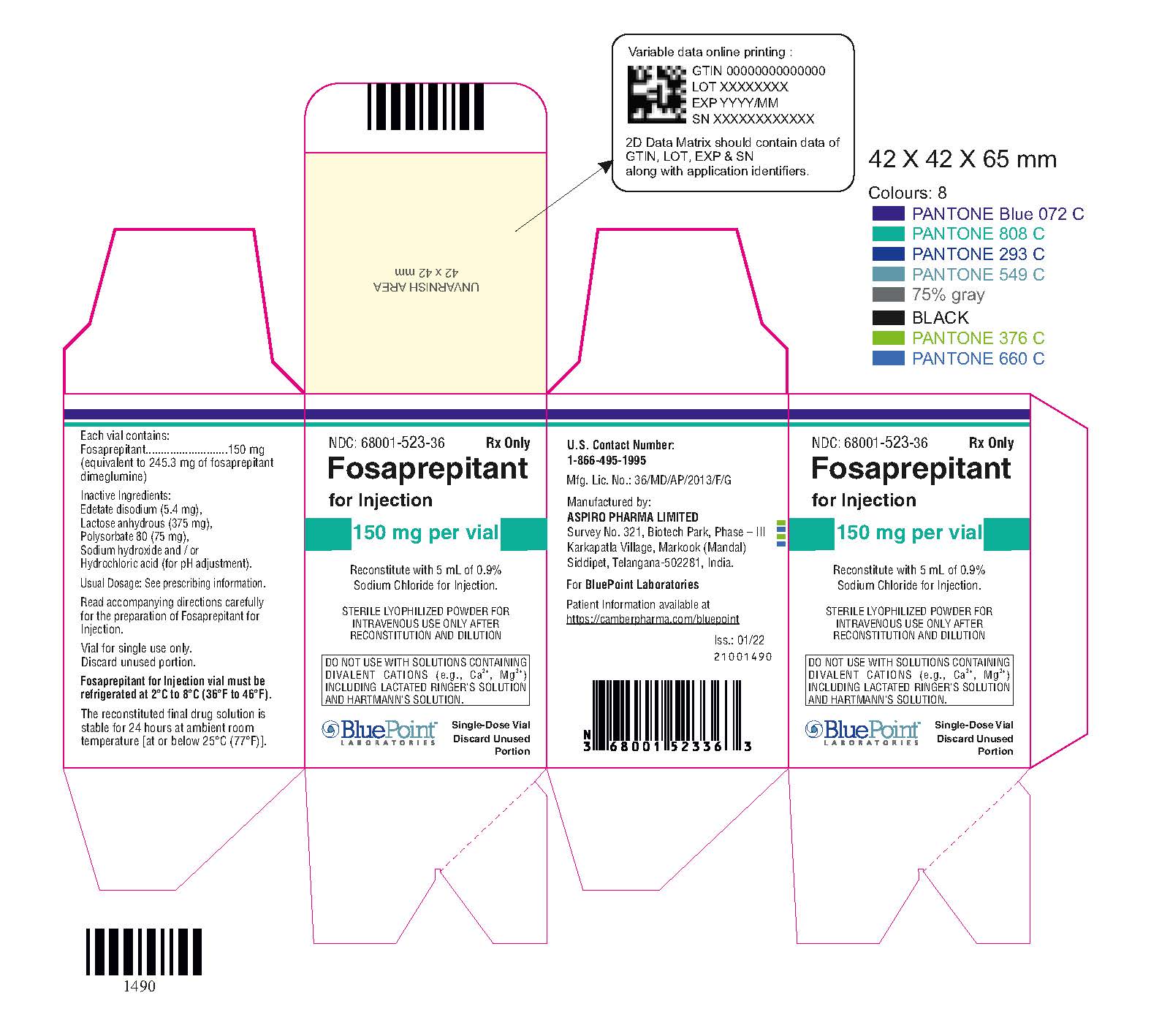
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

 Fosaprepitant
for Injection 150 mg per vial - Vial Carton Label
Fosaprepitant
for Injection 150 mg per vial - Vial Carton Label
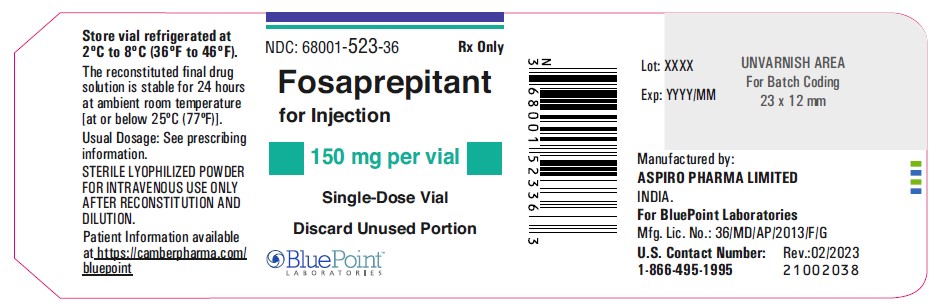
Fosaprepitant for Injection 150 mg per vial - Vial Label
DESCRIPTION SECTION
11 DESCRIPTION
Fosaprepitant for injection is a sterile, lyophilized formulation containing
fosaprepitant dimeglumine, a prodrug of aprepitant, a substance P/neurokinin-1
(NK 1) receptor antagonist, an antiemetic agent, chemically described as
1-Deoxy-1-(methylamino)-D-glucitol[3-[[(2 R,3 S)-2-[(1 R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-3-(4-fluorophenyl)-4-morpholinyl]methyl]-2,5-dihydro-5-oxo-1
H-1,2,4-triazol-1-yl]phosphonate (2:1) (salt).
Its empirical formula is C 23H 22F 7N 4O 6P ⋅ 2(C 7H 17NO 5) and its
structural formula is:
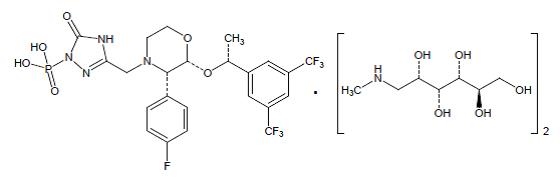
Fosaprepitant dimeglumine is a white to light brown powder with a molecular
weight of 1004.83. It is soluble in water and methanol.
Each vial of fosaprepitant for injection for administration as an intravenous
infusion contains 150 mg of fosaprepitant (equivalent to 245.3 mg of
fosaprepitant dimeglumine) and the following inactive ingredients: edetate
disodium (5.4 mg), lactose anhydrous (375 mg), polysorbate 80 (75 mg), sodium
hydroxide and/or hydrochloric acid (for pH adjustment).
