Alhemo
These highlights do not include all the information needed to use Alhemo safely and effectively. See full prescribing information for Alhemo.Alhemo (concizumab-mtci) injection, for subcutaneous useInitial U.S. Approval: 2024
156f6404-1f6f-417f-bcf8-f07a27a82cc1
HUMAN PRESCRIPTION DRUG LABEL
Jul 1, 2025
Novo Nordisk
DUNS: 622920320
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
concizumab
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
concizumab
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
concizumab
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 300 mg
NDC 0169-2081-03 List : 208103
**Alhemo****®**
**(concizumab-mtci) injection**
300 mg/3 mL(100 mg/mL)
Prefilled Pen
For subcutaneous use only
1x3 mL single-patient use prefilled pen
Dials in 1 mg increments and contains
300 mg total
Rx only
ATTENTION: Dispense the enclosed Medication Guide to each patient.

INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
Alhemo is indicated for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adult and pediatric patients 12 years of age and older with:
•
hemophilia A (congenital factor VIII deficiency) with or without FVIII inhibitors
•
hemophilia B (congenital factor IX deficiency) with or without FIX inhibitors
Alhemo is a tissue factor pathway inhibitor (TFPI) antagonist indicated for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adult and pediatric patients 12 years of age and older with:
•
hemophilia A (congenital factor VIII deficiency) with or without FVIII inhibitors
•
hemophilia B (congenital factor IX deficiency) with or without FIX inhibitors (1)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
Alhemo is contraindicated in patients with a history of known serious hypersensitivity to Alhemo or its components or the inactive ingredients [see Warnings and Precautions (5.1)and Description (11)].
Alhemo is contraindicated in patients with a history of known serious hypersensitivity to Alhemo or its components or the inactive ingredients. (4)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Thromboembolic Events
Alhemo may cause thromboembolic events. Venous and arterial thromboembolic events were reported in 1.9% of patients (6/320) in Alhemo clinical trials. These cases occurred in patients with multiple risk factors for thromboembolism, including the use of high doses or prolonged treatment with factor product or bypassing agent (2 of 6 patients).
Risk factors for thromboembolism may include the use of high and/or frequent doses of breakthrough bleed treatments (factor products or bypassing agents) or conditions in which tissue factor is overexpressed (e.g., atherosclerotic disease, crush injury, cancer, disseminated intravascular coagulation, thrombotic microangiopathy, or septicemia).
Inform Alhemo treated patients of signs and symptoms of thromboembolic events. Monitor patients for thromboembolic events. In case of suspicion of thromboembolic events, discontinue Alhemo and initiate further investigations and management strategies.
5.2 Hypersensitivity Reactions
Alhemo can cause hypersensitivity reaction, including serious cases. Alhemo is contraindicated in patients with a history of known serious hypersensitivity to Alhemo or its components or the inactive ingredients. Hypersensitivity reactions including erythema, rash, pruritus, and abdominal pain have occurred in Alhemo treated patients. One patient (less than 1% of patients treated in the clinical studies) experienced anaphylaxis which resolved after treatment with antihistamines and corticosteroids. Instruct patients of the signs of acute hypersensitivity reactions. Instruct patients to contact their healthcare provider for mild reactions and to seek urgent medical attention for moderate to severe reactions. Discontinue Alhemo if severe hypersensitivity symptoms occur and initiate medical management.
5.3 Increased Laboratory Values of Fibrin D-dimer and Prothrombin Fragment
1.2
Alhemo can cause increased levels of fibrin D-dimer and prothrombin fragment 1.2. Increased levels of fibrin D-dimer and increased levels of prothrombin fragment 1.2 were seen in 29 (9.1%) and 26 (8.1%) of patients, respectively. The plasma concentration of concizumab-mtci is positively correlated with fibrin D-dimer and prothrombin fragments 1.2 indicating a hemostatic effect of concizumab-mtci. For patients taking Alhemo, these coagulation biomarkers may not be reliable predictive markers for clinical decision-making with suspicion of thrombosis such as deep vein thrombosis (DVT) and pulmonary embolism (PE).
•
Thromboembolic Events: Monitor patients for thromboembolic events. Advise patients to report signs and symptoms, and if they occur discontinue prophylaxis. (5.1)
•
Hypersensitivity Reactions: In the event of a severe hypersensitivity reaction, discontinue Alhemo. (5.2)
•
Increased Laboratory Values of Fibrin D dimer and Prothrombin Fragment 1.2: Alhemo increases values of fibrin D dimer and prothrombin fragment 1.2. (5.3)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed in greater detail in other sections of the labeling:
•
Thromboembolic Events [see Warnings and Precautions (5.1)]
•
Hypersensitivity Reactions [see Warnings and Precautions (5.2)]
•
Increased Laboratory Values of Fibrin D-dimer and Prothrombin Fragment 1.2 [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in clinical practice.
The data in the WARNINGS AND PRECAUTIONS reflect exposure to Alhemo based on pooled data from clinical trials explorer3 (phase 1b), explorer4 (phase 2), explorer5 (phase 2), explorer7 (phase 3) and explorer8 (phase 3), in which a total of 320 male patients with hemophilia A with and without inhibitors and hemophilia B with and without inhibitors received at least one dose of Alhemo as routine prophylaxis. The patients were exposed for a total of 475 exposure years. Patients with HAwI (hemophilia A with inhibitors) and HBwI (hemophilia B with inhibitors)
The data described below reflect exposure of 52 patients with HAwI and HBwI who were previously treated on-demand therapy and
who were randomized in explorer7 to arm 1 to receive on- demand treatment with bypassing agents (n = 19) or arm 2 to receive
Alhemo prophylaxis (n = 33) at the recommended dosing regimen [see Clinical Studies (14.1)]. The median duration of treatment was
31.1 weeks (range 3.9, 72.9 weeks) in arm 1 (on-demand arm) and 40.1 weeks (range 3.1, 56.3 weeks) in arm 2 (Alhemo
prophylaxis).
Serious adverse reactions were reported in 6.1% of patients who received Alhemo. These serious adverse reactions were renal infarct
and hypersensitivity reaction. Permanent discontinuation of Alhemo due to an adverse reaction occurred in 1 patient due to a renal
infarct. Dosage interruptions of Alhemo due to an adverse reaction occurred in 1 patient (3%) and was a hypersensitivity reaction. The
most common adverse reactions (≥5%) were injection site reactions and urticaria (seeTable 1).
Table 1. Adverse Reactions Reported in ≥5% HAwI and HBwI Patients Randomized to Alhemo in Explorer7
|
Adverse Reaction |
Alhemo Prophylaxis N=33 (%) |
On-demand Treatment N=19 (%) |
|
Injection site reactions |
18% |
0% |
|
Urticaria |
6% |
0% |
Injection site reactions included: Injection site bruising, Injection site erythema, Injection site hematoma, Injection site hemorrhage, Injection site reaction and Injection site urticaria.
Urticaria included: Urticaria and Injection site urticaria.
Patients with HA (hemophilia A without inhibitors) and HB (hemophilia B without inhibitors)
The data described below reflect exposure of 63 patients with HA and HB who were previously treated on-demand therapy and who were randomized in explorer8 to arm 1 to receive on- demand treatment with factor product (n = 21) or arm 2 to receive Alhemo prophylaxis (n = 42) at the recommended dosing regimen [see Clinical Studies (14.2)]. The median duration of treatment was 24.1 weeks (range 23.6, 56.1 weeks) in arm 1 (on- demand arm) and 32.1 weeks (range 3.9, 33.6 weeks) in arm 2 (Alhemo prophylaxis).
The most common adverse reactions (≥5%) were injection site reactions and headache (seeTable 2).
Table 2. Adverse Reactions Reported in ≥5% HA and HB Patients Randomized to Alhemo in Explorer8
|
Adverse Reaction |
Alhemo Prophylaxis N=42 (%) |
On-demand Treatment N=21 (%) |
|
Injection site reactions |
7% |
0% |
|
Headache |
7% |
0% |
Injection site reactions included: injection site reaction, injection site rash, and injection site nodule
The most frequently reported adverse reactions (incidence ≥5%) were injection site reactions, headache and urticaria. (6.1)
**To report SUSPECTED ADVERSE REACTIONS, contactNovo Nordisk Inc. at 1-800-727-6500 or FDA at 1-800-FDA-1088 or **www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Breakthrough Bleeding Treatment
Take appropriate precautions when treating breakthrough bleeding events in hemophilia patients receiving Alhemo prophylaxis and FVIII or FIX or a bypassing agent [see Dosage and Administration (2.1)]. For mild and moderate bleeds that require additional treatment with FVIII or FIX or bypassing agents (e.g., rFVIIa or aPCC), the lowest-approved dose in the approved product labeling is recommended. For aPCC, a maximum dose of 100 units/kg body weight within 24 hours is recommended. For severe bleeds, follow the dosing instructions provided in the approved labeling for the specific product based on clinical judgement.
Additive and sometimes synergistic increase in thrombin peak as quantified in the thrombin generation assay has been observed in plasma from hemophilia patients who were on prophylactic treatment with concizumab-mtci with concomitant presence of rFVIII, rFIX or bypassing agents including rFVIIa and aPCC [see Clinical Pharmacology (12.2)].
Breakthrough Bleeding Treatment: While treatment with all bypassing agents (e.g., rFVIIa or aPCC) can be used for breakthrough bleeds, high and/or frequent doses of FVIII, FIX, or bypassing agents with Alhemo increases the risk of thromboembolism. (7.1)
INSTRUCTIONS FOR USE SECTION
Instructions for Use – 3.0 mL (300 mg)
INSTRUCTIONS FOR USE
Alhemo**®**** (al-HEE-mo)**
(concizumab-mtci)
injection, for subcutaneous use
300 mg/3 mL (100 mg/mL) single-patient-use prefilled pen
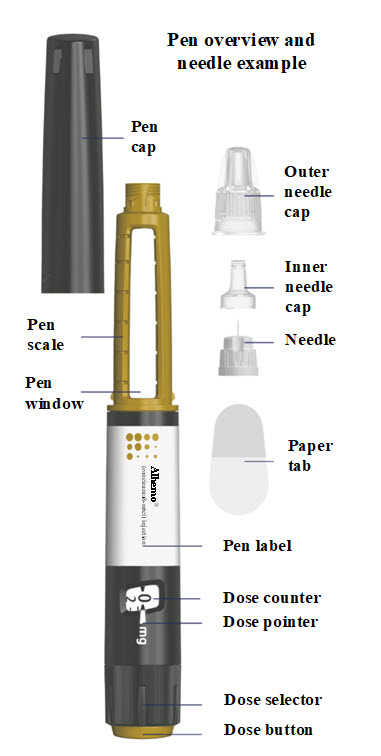
This Instructions for Use contains information on how to inject Alhemo. Read and understand these instructions before you use the Alhemo pen. Make sure you have received training from your healthcare provider before you inject with this pen for the first time.
Important information you need to know before injecting Alhemo
•
Use Alhemo exactly as prescribed by your healthcare provider.
•
Alhemo is supplied as a prefilled pen (called “Alhemo pen” or “pen” in these instructions). It contains 300 milligrams (mg) of Alhemo for subcutaneous injection. The pen contains multiple doses of Alhemo.
•
Your Alhemo pen is for single-patient-use only. Do not share your Alhemo pen with other people, even if the needle has been changed. You may give other people a serious infection or get a serious infection from them.
•
Your healthcare provider should show you or your caregiver how to use Alhemo before you use it for the first time.
•
Ask your healthcare provider if you need to use a different injection technique. For example, children and people who are lean may need to inject into a pinched fold of skin to avoid injecting too deep (into the muscle).
•
After 28 days, you must throw away (discard) your pen in an FDA-cleared sharps disposal container even if some medicine is left in the pen.
•
The pen can deliver doses from 1 mg to 80 mg in one injection. The interval on the dose counter is 1 mg. If you need more than 80 mg, you need to inject multiple times. Your healthcare provider will tell you how much Alhemo to inject.
Important information about needles
•
Needles can be used for 1 injection only. Always use a new needle for each injection.
•
Do not re-use needles as this reduces the risk of contamination, infection, leakage, blocked needles, and incorrect dosing.
•
Never share needles with others.
•
Alhemo is recommended to be used with NovoFine® and NovoFine® Plus 32G x 4 mm injection needles.
•
If you use needles longer than 4 mm, talk to your healthcare provider about how to perform your injection.
•
Talk to your healthcare provider or pharmacist for more information about needles for your Alhemo pen.
•
Do not use the needle if it is bent or damaged.
Gather the following supplies:
•
1 Alhemo pen
Supplies you will need that are not included in the carton:
•
a new needle for each injection. The Alhemo pen is recommended to be used with NovoFine® and NovoFine® Plus needles with a gauge of 32 and length of 4 mm (32G x 4 mm).
•
sharps disposal container. See** “Throwing away (disposing of) Alhemo pens, needles and needle caps.”**
•
alcohol swab
Where on my body should I inject my dose?
•
You can inject into the skin of:
o
your stomach-area (abdomen) at least 2 inches from your belly button (navel)
**or**
o
your upper legs (thigh).
•
The gray areas on the picture to the right show the injection sites.
•
Change (rotate) your injection site with each injection every day.**Do not** use the same site for each injection.
•
**Do not** inject into skin that is tender, bruised, red or hard, or areas where there are moles, scars, or stretch marks.
1. Check your Alhemo pen
•
**Wash your hands with soap and water**
Dry them well.
•
**Check the pen label**
Check the name, strength, and colored label to make sure you have the right medicine.
•
**Check the expiration date**
Check the expiration date (EXP) on the pen label to make sure it has not passed (YYYY-MM-DD). If the expiration date has passed, do not use the pen.
•
**Inspect the medicine**
Pull off the pen cap and check that Alhemo in the pen window is almost clear and colorless to slightly yellow. The medicine may contain particles that you can see through or are white.**Do not**use the pen if the medicine is discolored.
•
**If your pen is cold**
You can inject Alhemo right from the refrigerator or let it reach room temperature before you inject. You can warm the pen in the palms of your hands. Do not use any other heating sources.
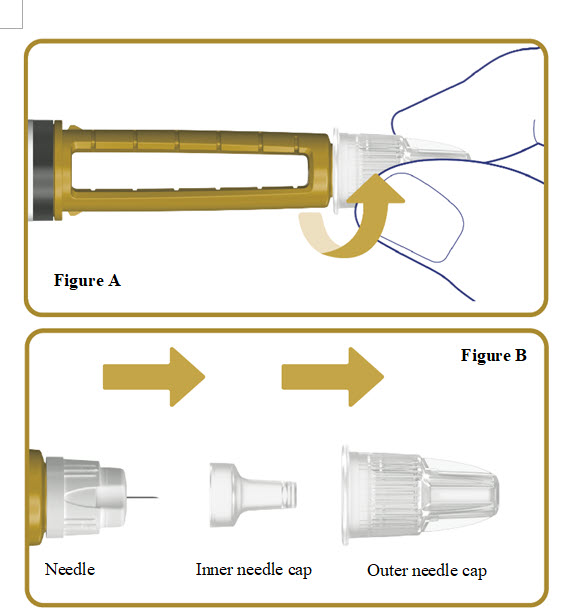
2. Attach a new needle
•
Take a new needle and tear off the paper tab.
•
Push the needle straight onto your pen. Turn the needle to the right (clockwise) until it is
on tight. See**Figure A**.
•
Pull off the outer needle cap. See**Figure B**.
•
Pull off the inner needle cap.See**Figure B**.
•
Throw the needle caps away in a FDA-cleared sharps disposal container.
Note: Always use a new needle for each injection.
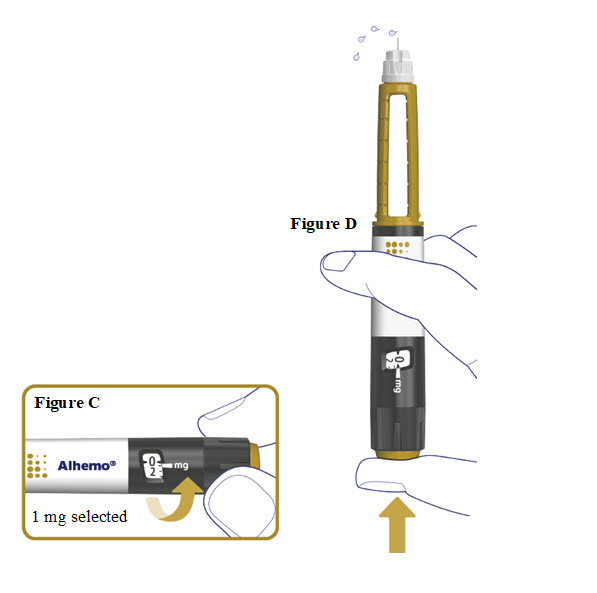
3. Prime before each dose. Dial to “1” and test the flow before each dose
•
A drop of Alhemo may appear at the needle tip, but you should still test the Alhemo flow before**each injection** to make sure you get the correct dose of Alhemo:
o
Turn the dose selector one marking to select 1 mg. See**Figure C**.
o
Press the dose button. See**Figure D**.
o
Watch a stream of Alhemo leaving the needle tip. See**Figure D**.
•
If no stream appears, go to**“Troubleshooting if no stream appears when testing the flow.”**
4. Select your dose
•
Turn the dose selector to select your prescribed dose.
•
You can adjust your dose by turning the dose selector in either direction (forwards or backwards).
•
If you need a larger dose than you can dial, you must inject yourself multiple times to get your full dose. For more information, see**Step 6**.
•
Each pen contains 300 mg of Alhemo.
•
The pen can deliver doses from 1 mg to 80 mg in one injection.
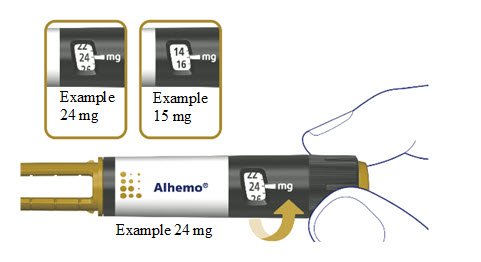
5a. Prepare the injection site
ReadSteps 5a and 5b before you start injecting. This is to make sure you get your full dose.
•
Select the injection site. See “**Where on my body should I inject my dose?”**
•
Wipe the injection site with an alcohol swab and let the area dry. Do not touch the injection site after cleaning.
•
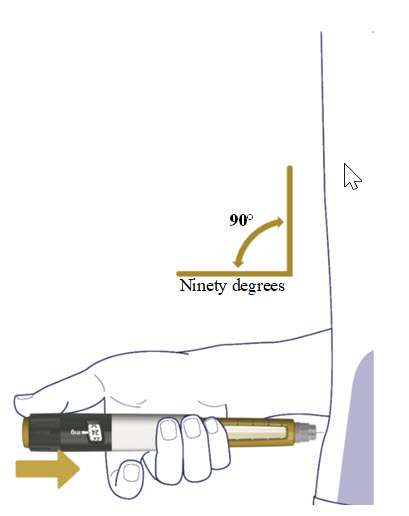
Insert the needle straight into your stomach-area (abdomen) or upper legs (thigh) at a 90-degree angle, as instructed by your healthcare provider.
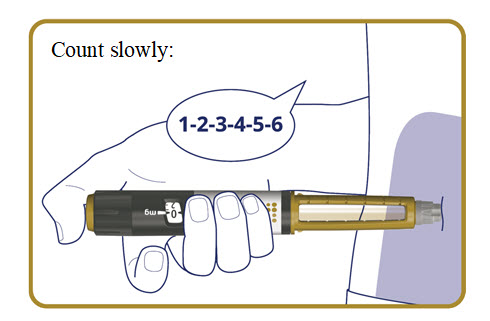
5b. Inject Alhemo
•
Press and hold the dose button down until the dose counter returns to**“0”**.
•
After the dose counter has returned to “**0**”,** count slowly to 6 while the needle is still in your skin.**
•
Remove the needle from your skin.
The pen clicks during the injection and you might also hear or feel a click when the dose counter returns to “0”.
6. Remove the needle
•
Carefully remove the needle from your pen by turning the needle to the left (counterclockwise). Do not touch the pen needle on either side to avoid sticking yourself with the needle.
•
Place the needle in an FDA-cleared sharps disposal container right away to reduce the risk of a needle stick. See**“Throwing away (disposing of) Alhemo pens, needles and needle caps.”**
•
**Do not**put the needle cap back on.
Do you need a larger dose than you can dial?
RepeatSteps 1 to** 6until you have received your full dose. When you have received your full dose go toStep 7**.
•
Use a new needle for each injection.
•
Test the Alhemo flow before each injection.
•
Make sure you know how much to inject in each injection to receive your full dose.
**Note:** Only split your dose if you have been trained or told by your healthcare provider on how to do this.
7. Recap the pen
•
Put the pen cap back on your pen to protect Alhemo from light.
•
Now your pen is ready for storage until you need it next time. See**“Storing Alhemo.”**

Troubleshooting if no stream appears when testing the flow (Step 3)
•
If no stream appears, repeat**Step 3** up to 6 times until you see a stream.
•
If still no stream appears, prepare a new needle (**Step 2**) and test again (**Step 3**).
•
If still no stream appears after attaching a new needle, do not use the pen. Use a new pen or call Novo Nordisk at 1-844-668-6732 for help.
How much Alhemo is left in your pen?
The pen scale shows about how much Alhemo is left in your pen. In the example below, 200 milligrams (mg) is remaining in the pen.
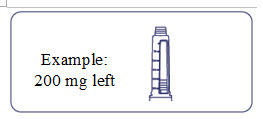
If you want to see more accurately how much Alhemo is left in your pen, turn the dose selector until it stops. The dose pointer will line up with the number of mg left in the pen.
If the dose counter shows 80 mg, at least 80 mg is left in the pen. If the dose counter shows less than 80 mg, the number shown in the dose counter is the number of mg left in your pen. In the example below, the dose counter shows 34 mg, so there is 34 mg of Alhemo left in the pen.
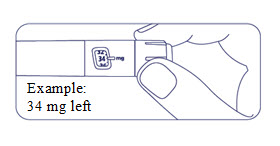
Storing Alhemo
•
**Before first use:**
o
Store unused Alhemo pens in the refrigerator between 36°F to 46°F (2°C to 8°C).
•
**After first use:**
o
Store the Alhemo pen in the refrigerator between 36°F to 46°F (2°C to 8°C) or at room temperature no warmer than 86°F (30°C) for up to 28 days.
o
Write the date of first use in the space provided on the carton.
o
Throw away (discard) the Alhemo pen 28 days after first opening even if some medicine is left in the pen.
•
Store Alhemo with the cap on and keep it in the original carton to protect from light.
•
Do not store Alhemo in direct sunlight and keep away from direct heat.
•
When stored in the refrigerator, do not store the pen directly next to the cooling element (the part that cools the refrigerator).
•
Do not freeze Alhemo.
•
Do not use Alhemo if it has been frozen or if it has been stored above 86°F (30°C).
Keep Alhemo and all medicine out of the reach of children.
Throwing away (disposing of) Alhemo pens, needles and needle caps
•
Put your used pens, needles, and needle caps in an FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and pens in your household trash.
•
If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
o
made of heavy-duty plastic,
o
can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
o
upright and stable during use,
o
leak-resistant, and
o
properly labelled to warn of hazardous waste inside the container.
•
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should dispose of used needles and pens. For more information about safe sharps disposal, and for specific information about safe sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
•
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
Scan the QR code below to access the Instructions for Use or visit: www.Alhemo.com

Patent Information: http://novonordisk-us.com/products/product- patents.html
Alhemo® is a registered trademark of Novo Nordisk Health Care AG.
NovoFine®, NovoFine® Plus, and Novo Nordisk® are registered trademarks of Novo Nordisk A/S.
© 2025 Novo Nordisk Health Care AG
For further information contact:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536, USA
1-844-668-6732
Manufactured by:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536
U.S. License No. 1261
At: Novo Nordisk A/S
Novo Allé 1
2880 Bagsvaerd
Denmark
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: 07/2025
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on its mechanism of action, Alhemo may cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. There are no available data on Alhemo use in pregnant women to evaluate for a drug- associated risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes. Animal reproduction studies have not been conducted with Alhemo. Although there are no data on concizumab-mtci, monoclonal antibodies can be actively transported across the placenta, and concizumab-mtci may cause fetal harm. It is unknown whether Alhemo can affect reproductive capacity. Alhemo should only be used during pregnancy if the potential benefit for the mother outweighs the potential risk to the fetus.
The estimated background risk of birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defect and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of Alhemo in either human or animal milk, the effect on the breastfed child, or the effects on milk production.
Clinical Considerations
Human IgGs are known to be excreted in breast milk during the first few days after birth, decreasing to low concentrations soon afterwards; consequently, a risk to the breast-fed infant cannot be excluded during this short period. Afterwards, Alhemo could be used during breast-feeding if clinically needed.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Pregnancy testing is recommended for females of reproductive potential.
Contraception
Women of childbearing potential should use a highly effective form of contraception during treatment with Alhemo and for 7 weeks after ending treatment. The benefits and thromboembolic risks of the type of contraceptives used should be evaluated by the treating physician.
8.4 Pediatric Use
The safety and effectiveness of Alhemo for hemophilia A and B with and without inhibitors have been established in pediatric patients aged 12 years and older. Use of Alhemo for this indication is supported by evidence from adequate and well-controlled studies in adult and pediatric patients aged 12 years and older [see Adverse Reactions (6.1) and Clinical Studies (14)]. The safety and effectiveness of Alhemo for hemophilia A and B with and without inhibitors have not been established in pediatric patients younger than 12 years of age.
8.5 Geriatric Use
Clinical studies of Alhemo did not include sufficient numbers of patients 65 years of age and older to determine whether they respond differently from younger adult patients.
8.6 Body Weight
The exposure of concizumab-mtci increased with increasing body weight [see Clinical Pharmacology (12.3)]. However, patients should receive the approved recommended Alhemo dosage titration and concizumab-mtci plasma concentration monitoring regardless of body weight [see Dosage and Administration (2.1)].
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Hemophilia A and B with Inhibitors in Patients ≥12 Years of Age
(Explorer7)
The efficacy of Alhemo in patients age 12 years and older with hemophilia A and B with inhibitors was evaluated in the explorer7 trial (NCT04083781). The explorer7 trial was a multinational, multicenter, open-label, randomized trial that investigated the safety and efficacy of Alhemo for routine prophylaxis in 91 adults (58 HAwI and 33 HBwI) and 42 adolescents (22 HAwI and 20 HBwI) male patients with hemophilia A or B with inhibitors who have been prescribed, or are in need of, treatment with bypassing agents. Eptacog alfa was the rFVIIa used in explorer7. Patients were excluded if they had a history, current signs or symptoms, or at high risk of thromboembolic events, ongoing or planned immune tolerance induction treatment, in addition to patients with planned major surgery.
Among the 133 patients included in the trial the mean age was 29 years (range: 12 to 79); 42 patients were 12 to <18 years of age, 89 patients were 18 to 64 years of age, and 2 patients were ≥65 years of age, and all were male. Seventy-eight (78) patients were White, 37 patients were Asian, 9 patients were Black or African American, 6 patients had race information unreported, and 3 patients were American Indian or Alaska Native; 6 patients identified as Hispanic or Latino, 122 patients identified as not Hispanic or Latino and 5 patients had ethnicity information unreported.
The trial was comprised of 4 arms, two randomized arms and two nonrandomized arms:
•
Arms 1 and 2: 52 patients (27 HAwI, and 25 HBwI), previously treated on-demand, were randomized 1:2 to no prophylaxis (arm 1: on demand treatment with bypassing agents) or Alhemo prophylaxis (arm 2), with stratification by hemophilia type (HAwI, HBwI) and prior 24-week bleeding rate (< 9 or ≥9)
•
Arms 3 and 4: 81 additional patients (53 HAwI and 28 HBwI) treated with Alhemo prophylaxis
Treatment with Alhemo included a loading dose of 1 mg/kg by subcutaneous injection on the first day and a once daily dose of 0.2 mg/kg by subcutaneous injection starting on the second day. The dose was individualized to 0.25 mg/kg or 0.15 mg/kg if Alhemo plasma concentration measured once after 4 weeks of treatment was <200 ng/mL or >4000 ng/mL, respectively. Measurement of concizumab-mtci plasma concentration after 4 weeks was used to optimize the daily maintenance dose. In the trial, a total of 108 patients received their individualized dose, 1 patient on 0.15 mg/kg, 79 patients on 0.2 mg/kg and 28 patients on 0.25 mg/kg.
Efficacy was evaluated in hemophilia A and B patients with inhibitors when all patients in arms 1 and 2 had completed at least 24 or at least 32 weeks, respectively, by comparing the number of treated bleeding episodes between Alhemo prophylaxis (arm 2) and no prophylaxis (arm 1). Using a negative binomial model, a ratio of the annualized bleeding rates (ABR) was estimated to 0.14 (p<0.001), corresponding to a reduction in ABR of 86% for subjects on Alhemo prophylaxis compared to no prophylaxis. The estimated mean ABR was 1.7 [95%CI: 1.01; 2.87] for patients on Alhemo prophylaxis (arm 2) and 11.8 [95%CI: 7.03; 19.86] for patients on no prophylaxis (arm 1).
14.2 Hemophilia A and B without Inhibitors in Patients ≥12 Years of Age
(Explorer8)
The efficacy of Alhemo in patients 12 years of age and older with hemophilia A and B without inhibitors was established by the explorer8 trial (NCT04082429). The explorer8 trial was an interventional, multinational, multicenter, open- label, randomized trial to investigate efficacy and safety of Alhemo for the prophylaxis treatment of bleeding episodes in 118 adult (67 hemophilia A and 51 hemophilia B) and 38 adolescent (23 hemophilia A and 15 hemophilia B) male patients with hemophilia A or B without inhibitors.
Among the 156 patients included in the trial the mean age was 32 years (range: 12 to 72); 38 patients were 12 to <18 years of age, 114 patients were 18 to 64 years of age, and 4 patients were ≥65 years of age, and all were male. One- hundred-three (103) patients were White, 44 patients were Asian, 4 patients were Black or African American, 3 patients were American Indian or Alaska Native and 2 patients had race information unreported; 12 patients identified as Hispanic or Latino, 143 patients identified as not Hispanic or Latino and 1 patient had ethnicity information unreported.
The trial included two randomized arms:
•
Arms 1 and 2: 63 patients (27 hemophilia A and 36 hemophilia B), previously treated on-demand, were randomized 1:2 to no prophylaxis (on demand FVIII/FIX, arm 1) or Alhemo prophylaxis (arm 2), with stratification by hemophilia type (hemophilia A, hemophilia B) and prior 24-week bleeding rate (< 9 or ≥ 9)
Treatment with Alhemo included a loading dose of 1 mg/kg by subcutaneous injection on the first day and a once daily dose of 0.2 mg/kg by subcutaneous injection starting on the second day. The dose was individualized to 0.25 mg/kg or 0.15 mg/kg if Alhemo plasma concentration measured once after 4 weeks of treatment was <200 ng/mL or >4000 ng/mL, respectively. Measurement of concizumab-mtci plasma concentration after 4 weeks was used to optimize the daily maintenance dose. In the trial, a total of 138 patients received their individualized dose, 10 patients on 0.15 mg/kg, 93 patients on 0.2 mg/kg and 35 patients on 0.25 mg/kg.
Efficacy was evaluated separately in hemophilia A patients without inhibitors and in hemophilia B patients without inhibitors when all patients in arms 1 and 2 had completed the main part of the trial (at least 24 or at least 32 weeks, respectively), based on the number of treated bleeding episodes, comparing Alhemo prophylaxis with no prophylaxis.
Using a negative binomial model, a ratio of the annualized bleeding rates (ABR) was estimated to 0.14 (p< 0.001) for patients with hemophilia A, corresponding to a reduction in ABR of 86% for subjects on Alhemo prophylaxis compared to no prophylaxis. Using the same model for patients with hemophilia B, the ratio was estimated to 0.21 (p< 0.001), corresponding to a reduction in ABR of 79% for subjects on Alhemo prophylaxis compared to no prophylaxis. For patients with hemophilia A, the estimated mean ABR was 2.7 [95%CI: 1.63; 4.59] for patients on Alhemo prophylaxis (arm 2) and 19.3 [95%CI: 11.25; 33.03] for patients on no prophylaxis (arm 1). For patients with hemophilia B, the estimated mean ABR was 3.1 [95%CI: 1.91; 5.04] for patients on Alhemo prophylaxis (arm 2) and 14.8 [95%CI: 8.14; 26.86] for patients on no prophylaxis (arm 1).
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
For subcutaneous use only.
Alhemo should be administered once daily. Avoid missed doses.
Recommended dosing regimen:
•
Day 1: Loading dose of 1 mg/kg
•
Day 2: Once daily dose of 0.2 mg/kg until individualization of maintenance dose (see below)
•
4 weeks after initiation of treatment: For dose optimization measure concizumab-mtci plasma concentration by Concizumab Enzyme-Linked Immunosorbent Assay (ELISA) prior to administration of next scheduled dose using an FDA-authorized test. Information on the FDA-authorized test for the measurement of concizumab-mtci plasma concentration is available at https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/denovo.cfm. Once the concizumab-mtci concentration result is available, individualize the maintenance dose of Alhemo no later than 8 weeks after initiation of treatment, based on the following concizumab-mtci plasma concentrations:
o
Less than 200 ng/mL: adjust to a once daily dose of 0.25 mg/kg
o
200 to 4,000 ng/mL: continue once daily dose of 0.2 mg/kg
o
Greater than 4,000 ng/mL: adjust to a once daily dose of 0.15 mg/kg
The calculated dose is rounded off to the nearest injectable dose as follows:
•
60 mg/1.5 mL (40 mg/mL) in increments of 0.4 mg (brown label)
•
150 mg/1.5 mL (100 mg/mL) in increments of 1 mg (gold label)
•
300 mg/3 mL (100 mg/mL) in increments of 1 mg (white label)
Additional measurements of concizumab-mtci plasma concentration should be taken at routine clinical follow-ups provided the patient has been on the same maintenance dose for 8 weeks of treatment to ensure steady state plasma concentration. Maintenance of concizumab plasma concentration above 200 ng/mL is important to decrease the risk of bleeding episodes. If concizumab-mtci plasma concentration remains below 200 ng/mL at two consecutive measurements, evaluate the benefits of continued Alhemo treatment versus the potential risk of bleeding events, and consider alternative therapies if available.
As Alhemo is dosed by body weight (mg/kg), it is important to recalculate the dose when patients experience body weight changes.
Missed Dose
Adherence to daily dosing of Alhemo is important to maintain protection against bleeding. This is especially important during the initial 4 weeks of treatment to ensure a correct maintenance dose is established. Patients who miss a dose during the initial 4-week period should inform their healthcare professional and resume once daily dosing at the initial 0.2 mg/kg dose level.
Missed Doses Once the Maintenance Dose Has Been Established
The following dosing guidelines should applyONLY when a patient has forgotten to or neglected to take their once daily maintenance dose:
•
1 missed dose: Resume once daily treatment at the maintenance dose level
•
2 to 6 missed doses: Resume treatment with a double dose followed by once daily treatment at the maintenance dose level
•
7 or more missed doses: Physician should be contacted, and a new loading dose should be considered [see Dosage and Administration (2.1)]
Management of Breakthrough Bleeds
No dose adjustment of Alhemo is required in the case of breakthrough bleeds.
Management in the Perioperative Setting
No dose adjustment of Alhemo is required in the case of minor surgeries.
As there is limited experience in the perioperative setting, it is generally recommended to pause Alhemo at least 4 days prior to major surgery. Alhemo therapy can be resumed 10-14 days after surgery on the same maintenance dose without a new loading dose, considering the overall clinical picture of the patient. If necessary, consult a physician experienced in surgery of patients with bleeding disorders.
Immune Tolerance Induction
The safety and efficacy of concomitant use of Alhemo in patients receiving ongoing Immune Tolerance Induction (ITI), a desensitization strategy for the eradication of inhibitors, have not been established, and no data are available. Careful assessment of the potential benefits and risks should be performed if continuation or initiation of Alhemo during ITI is considered.
2.2 Changing to Alhemo from Other Hemostatic Products
•
Discontinue treatment with rFVIIa at least 12 hours before starting Alhemo.
•
Discontinue treatment with activated prothrombin complex concentrate (aPCC) at least 48 hours before starting Alhemo.
•
Discontinue prophylactic use of standard half-life factor VIII (FVIII) or factor IX (FIX) at least 24 hours before starting Alhemo.
•
When changing from other products to Alhemo, the half-life of the previous product should be considered.
Healthcare providers should discuss with patients receiving Alhemo and/or their caregivers the dose and schedule of bypassing agents or FVIII or FIX, if required, while receiving Alhemo prophylaxis.
2.3 Instructions and Dosage Modification for Breakthrough Bleeding
Treatment with FVIII or FIX or all bypassing agents (e.g., rFVIIa or aPCC) can be used for breakthrough bleeds, and the dose and duration will depend on the location and severity of the bleed.
For mild and moderate bleeds that require additional treatment with FVIII or FIX or bypassing agents (e.g., rFVIIa or aPCC), the lowest-approved dose and the dose interval in the approved product labeling is recommended. For aPCC, a maximum dose of 100 units/kg body weight within 24 hours is recommended.
For severe bleeds, follow the dosing instructions provided in the approved labeling for the specific product based on clinical judgement.
2.4 Administration and Use Instructions
Treatment is intended for use under the guidance of a healthcare provider. Treatment should be initiated in a nonbleeding state.
Alhemo may be self-administered or administered by a caregiver after appropriate training and reading the Instructions for Use, if a healthcare provider determines that is appropriate.
Administer Alhemo by subcutaneous injection to the abdomen or thigh with rotation of injection site every day. Subcutaneous injections should not be given in areas where the skin is tender, bruised, red or hard, or areas where there are moles, scars, or stretch marks. Children and lean patients should be instructed to use injection techniques that minimize risk of intramuscular injection, e.g. injecting into a pinched fold of skin.
Always use a new needle for each injection.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Alhemo is a clear to slightly opalescent and colorless to slightly yellow solution that may contain translucent to white particles. Do not use if the solution is discolored.
Each Alhemo prefilled pen is for use by a single patient. An Alhemo pen must not be shared between patients, even if the needle is changed.
Alhemo is recommended to be used with NovoFine® or NovoFine® Plus needles with a gauge of 32 and a length of 4 mm. If needles longer than 4 mm are used, injection techniques that minimize the risk of intramuscular injection should be used.
Instructions for delivering the dosage are provided in the Instructions for Use leaflet enclosed with each Alhemo single-patient-use prefilled pen.
Administer Alhemo by subcutaneous injection to the abdomen or thigh with daily rotation of injection sites. (2.2)
Recommended dosing regimen:
•
Day 1: Loading dose of 1 mg/kg
•
Day 2: Once daily dose of 0.2 mg/kg until individualization of maintenance dose (2.1)
o
4 weeks after initiation of treatment: For dose optimization, measure concizumab‑mtci plasma concentration by Concizumab Enzyme-Linked Immunosorbent Assay (ELISA) prior to administration of next scheduled dose using an FDA-authorized test for the measurement of concizumab-mtci concentration in plasma.
See full Prescribing Information for important preparation and administration instructions and dosage adjustment. (2.1, 2.3, 2.4)
DESCRIPTION SECTION
11 DESCRIPTION
Concizumab-mtci, is a humanized IgG4 monoclonal antibody produced by recombinant DNA technology in Chinese Hamster Ovary (CHO) cells with an approximate molecular weight of 149 kDa.
Alhemo (concizumab-mtci) injection is a clear to slightly opalescent, and colorless to slightly yellow solution that may contain translucent to white particles. Alhemo is supplied as a single-patient-use prefilled pen for subcutaneous injection.
Each 1 mL of Alhemo single-patient-use prefilled pen (60 mg/1.5 mL) contains 40 mg active ingredient concizumab-mtci. Each 1 mL of Alhemo single-patient- use prefilled pen (150 mg/1.5 mL) contains 100 mg active ingredient concizumab-mtci. Each 1 mL of Alhemo single-patient-use prefilled pen (300 mg/3 mL) contains 100 mg active ingredient concizumab-mtci.
Each 1 mL of Alhemo single-patient-use prefilled pen contains the following excipients: arginine hydrochloride (5.27 mg), histidine (5.12 mg), phenol (3.5 mg), polysorbate 80 (0.25 mg), sodium chloride (1.46 mg), sucrose (51.3 mg), and water for injection. Hydrochloric acid and sodium hydroxide may be added to adjust the pH to 6.0.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Concizumab-mtci is a monoclonal antibody antagonist of endogenous Tissue Factor Pathway Inhibitor (TFPI). Through the inhibition of TFPI, concizumab- mtci acts to enhance FXa production during the initiation phase of coagulation which leads to improved thrombin generation and clot formation with the goal of achieving hemostasis in patients with Hemophilia A or B regardless of their inhibitor status.
The effect of concizumab-mtci is not influenced by the presence of inhibitory antibodies to FVIII or FIX. There is no structural relationship or sequence homology between concizumab-mtci and FVIII or FIX and, as such, treatment with concizumab-mtci does not induce or enhance the development of direct inhibitors to FVIII or FIX.
12.2 Pharmacodynamics
Increasing concizumab-mtci dose levels resulted in decreased levels of free TFPI (plasma TFPI not bound to concizumab-mtci) and increased duration of free TFPI suppression. In hemophilia patients with inhibitors, free TFPI plasma levels predose decreased from a geometric mean (CV%) of 88.3 (20%) ng/mL at baseline to 10.7 (105%) ng/mL at week 24 in patients on Alhemo prophylaxis, while the geometric mean (CV%) was 76 (18%) ng/mL at week 24 for patients treated on-demand. In hemophilia patients without inhibitors, free TFPI plasma levels predose decreased from a geometric mean (CV%) of 84.2 (19%) ng/mL at baseline to 11.1 (102%) ng/mL at week 24 in patients on Alhemo prophylaxis, while the geometric mean (CV%) was 80.6 (18%) ng/mL at week 24 for patients treated on-demand. Mean thrombin peak within the range of normal plasma reflected that concizumab‑mtci re‐established thrombin generation capacity.
Drug Interaction
In vitro studies
Additive and sometimes synergistic increase in thrombin peak as quantified in the thrombin generation assay has been observed in plasma from hemophilia patients who were on prophylactic treatment with concizumab-mtci with concomitant presence of rFVIII, rFIX or bypassing agents including rFVIIa and aPCC. Depending on concentrations of concizumab-mtci and hemostatic agents, the impact on thrombin peak ranged from being additive with all hemostatic agents to an extra 40% effect with rFVIIa, an extra 33% effect with aPCC, an extra 22% with rFVIII and less than 13% with rFIX.
12.3 Pharmacokinetics
Peak and trough geometric mean plasma concizumab-mtci concentrations at steady state are shown inTable 3. Following a single Alhemo loading dose of 1 mg/kg, the steady state concentrations were reached around Day 4 and remained within a stable exposure range with daily maintenance doses. Concizumab-mtci AUC and Cmax increased with increasing dose in a greater than dose- proportional manner following subcutaneous administration.
Table 3. Steady State Concizumab-mtci Concentrations During 24-hour Dosing Interval at Week 24
|
Parameters |
Hemophilia A and B without inhibitors All maintenance doses N=127****a |
HAwI and HBwI All maintenance doses N=99****a |
|
Cmax,ss (ng/mL), geometric mean (CV%) N |
893.4 (144.5%) N=103 |
1167.1 (128%) N=69 |
|
Ctrough,ss (ng/mL), geometric mean (CV%) N |
647.3 (178.8%) N=116 |
665.4 (221%) N=94 |
|
Cmax / Ctrough ratio, mean (SD) N |
1.5 (0.5) N=103 |
2.2 (5.2) N=69 |
Abbreviations: Cmax,ss, maximum plasma concentration at steady state; Ctrough,ss, trough plasma concentration at steady state; HAwI, hemophilia A with inhibitors; HBwI, hemophilia B with inhibitors.
a On concizumab-mtci dosing regimen.
Absorption
Concizumab-mtci time to maximum plasma concentration ranged from 8 hours to 99
hours (4.1 days) following a single Alhemo subcutaneous dose of 0.05 to 3
mg/kg in healthy and hemophilia subjects.
Distribution
Concizumab-mtci volume of distribution for a typical 70 kg patient is about 3 L.
Elimination
Concizumab-mtci is cleared via linear and nonlinear mechanisms. Concizumab- mtci exhibited nonlinear pharmacokinetics due to target-mediated drug disposition (TMDD) which occurs when it forms concizumab-mtci/TFPI complex. Once the target becomes saturated, linear pathway (i.e., catabolism) dominates.
Based on population pharmacokinetic analysis, 90% of concizumab-mtci is expected to be eliminated by the end of approximately 4 days after the last dose (time for 50% of drug to be eliminated is approximately 1 day).
Metabolism
Concizumab-mtci is expected to be metabolized into small peptides by catabolic pathways.
Specific Populations
No clinically significant differences in the pharmacokinetics of concizumab-
mtci were observed based on age (12 years to 79 years), race (White 64.3%,
Asian 25.9%, Black 4.9%), hemophilia type (A and B with or without
inhibitors). No dedicated studies have been conducted to evaluate the impact
of renal or hepatic impairment. As concizumab-mtci is a monoclonal antibody,
there is no expectation for concizumab-mtci exposures to be different in
patients with renal or hepatic impairment.
Body weight
The exposure of concizumab-mtci increased with increasing body weight (27 kg to 130.7 kg) [see Use in Specific Populations (8.6)].
12.6 Immunogenicity
The observed incidence of antidrug antibodies is highly dependent on the sensitivity and the specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of antidrug antibodies (ADA) in the studies described below with the incidence of ADA in other studies, including those of concizumab-mtci or of other concizumab products.
During the treatment period in 5 trials with a duration of 11 weeks, ≥76 weeks, ≥76 weeks, ≥56 weeks and ≥56 weeks, 71 out of the 320 treated patients (22.2%) developed anticoncizumab-mtci antibodies. Among the 71 patients who tested positive for ADA, 18 patients (25.4%) developed neutralizing antibodies (NAbs) against concizumab-mtci. In 2 patients (2.8%) who developed NAb against concizumab-mtci, free TFPI levels were restored to baseline indicative of effectiveness likely being compromised. In the remaining 69 patients (97.2%), there was no identified clinically significant effect of the antibodies on pharmacokinetics, pharmacodynamics, safety, or effectiveness of concizumab- mtci.
12.7 Interference of Concizumab-mtci with Laboratory Tests
In vitro studies
No clinically significant interference with standard prothrombin and activated partial thromboplastin time assays or FVIII or FIX activity measurement using clot and chromogenic assays was observed with concizumab-mtci. Further, no clinically relevant interference with assays for inhibitory antibodies to FVIII or FIX (Bethesda assay) was observed with concizumab-mtci.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term animal studies have been conducted to evaluate the carcinogenic potential of concizumab-mtci nor have studies been performed to determine the effects of concizumab-mtci on genotoxicity.
In a 26-week toxicity study in sexually mature male and female cynomolgus monkeys with subcutaneous doses up to 9 mg/kg/day (corresponding to 3400-fold the human exposure, based on AUC0 to 24h), concizumab-mtci did not affect fertility (testicular size, sperm functionality or menstrual cycle duration) and did not cause any changes in the male or female reproductive organs.
13.2 Animal Toxicology and/or Pharmacology
Pharmacology mediated formation of thrombi manifested in cynomolgus monkeys dosed subcutaneously during toxicology studies of 13-weeks (≥ 10 mg/kg/day), 26-weeks (≥ 3 mg/kg/day) and 52-weeks (≥ 1 mg/kg/day) in duration corresponding to ≥ 3732-fold, ≥ 955-fold and ≥ 308-fold, respectively, the human clinical exposure based on AUC0 to 24h in patients with Hemophilia A or B at the MRHD.
In a 28-day drug-drug interaction toxicity study in cynomolgus monkey with daily dosing of 1 mg/kg concizumab-mtci to achieve steady state, three consecutive intravenous doses of up to 1 mg/kg rFVIIa were administered with 2-hour intervals to the concizumab‑mtci dosed animals. No adverse findings were observed at a concizumab-mtci exposure corresponding to 205-fold the human exposure, based on AUC0 to 24h.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
Alhemo (concizumab-mtci) injection is a clear to slightly opalescent, colorless to slightly yellow liquid, that may contain translucent to white particles. Alhemo is available as one single-patient-use prefilled pen per carton in the following presentations (seeTable 4):
Table 4. Alhemo Presentations
|
Presentation |
Label |
NDC Number |
|
60 mg/1.5 mL (40 mg/mL) |
Brown |
0169-2084-15 |
|
150 mg/1.5 mL (100 mg/mL) |
Gold |
0169-2080-15 |
|
300 mg/3 mL (100 mg/mL) |
White |
0169-2081-03 |
Storage and Handling
Before first use: Store in a refrigerator at 36°F to 46°F (2°C to 8°C) in the original carton to protect from light. Do not freeze.
After first use: Store in a refrigerator at 36℉ to 46˚F (2℃ to 8˚C) or at room temperature below 86˚F (30˚C) for up to 28 days. Write the date of first use in the space provided on the carton. Discard the unused portion of the pen 28 days after first opening.
Store Alhemo with the cap on and in the original carton to protect from light. Alhemo should not be stored in direct sunlight, and the Alhemo pen should be kept away from direct heat. Do not freeze or store it close to a cooling element in a refrigerator (the part that cools the refrigerator). Do not use Alhemo if it has been frozen or stored at temperatures above 86˚F (30˚C).
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
•
Thromboembolic events: Inform patients of the signs and symptoms of thromboembolic events, including swelling, warmth, pain, or redness of the skin (and that these could be symptoms of a blood clot in the legs or arms); shortness of breath or severe chest pain (and that these could be symptoms of blood clots in the chest [heart and lungs]).; headache, confusion, difficulty with speech or movement, numbness of the face, eye pain or swelling, or vision problems (and that these could be symptoms of a blood clot in the brain or eyes); or sudden pain in the stomach or lumbar area (and that these could be symptoms of blood clots in the gut or kidneys). Advise patients to contact their healthcare provider for mild reactions and to seek urgent medical attention for moderate to severe reactions [see Warnings and Precautions (5.1)].
o
In conditions in which tissue factor is overexpressed (e.g., advanced atherosclerotic disease, crush injury, cancer, or septicemia), there may be a risk of thromboembolic events or disseminated intravascular coagulation (DIC) with Alhemo treatment.
•
Hypersensitivity: Inform patients of the early signs of hypersensitivity reactions, including rash, redness, hives, and itching, facial swelling, tightness of the chest, and wheezing as well the signs of an anaphylactic reaction such as itching on large areas of skin; redness and/or swelling of lips, tongue, face, or hands; difficulty swallowing; shortness of breath; wheezing; tightness of the chest; pale and cold skin; fast heartbeat; or dizziness/low blood pressure. Advise patients to discontinue use of Alhemo immediately and contact their healthcare provider if any signs or symptoms occur [see Warnings and Precautions (5.2)].
Advise patients on the importance of adherence to daily dosing and to speak with their healthcare provider before discontinuing treatment with Alhemo as patients who are not adhering to daily dosing or stop treatment with Alhemo may no longer be protected against bleeding.
Advise patients to follow the recommendations regarding proper sharps disposal provided in the FDA-approved Instructions for Use.
Patent Information: http://novonordisk-us.com/products/product- patents.html
Alhemo® is a registered trademark of Novo Nordisk Health Care AG.
NovoFine®, NovoFine® Plus, and Novo Nordisk® are registered trademarks of Novo Nordisk A/S.
For information about Alhemo contact:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536
1-844-668-6732
Manufactured by:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536
U.S. License No. 1261
At: Novo Nordisk A/S
Novo Allé 1
2880 Bagsvaerd
Denmark
© 2025 Novo Nordisk
SPL MEDGUIDE SECTION
Medication Guide
MEDICATION GUIDE
Alhemo**®**** (al-HEE-mo)**
(concizumab-mtci)
injection, for subcutaneous use
|
What is the most important information I should know about Alhemo? • • See**“How should I use Alhemo?”**for more information on how to use Alhemo. | |
|
What is Alhemo? Alhemo is a prescription medicine used for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adults and children 12 years of age and older with: • • It is not known if Alhemo is safe and effective in people while receiving ongoing Immune Tolerance Induction (ITI). It is not known if Alhemo is safe and effective for hemophilia A and B with and without inhibitors in children younger than 12 years of age. | |
|
Do not use Alhemo if youare allergic to concizumab-mtci or any of the ingredients in Alhemo. See the end of this Medication Guide for a complete list of ingredients in Alhemo. | |
|
Before using Alhemo, tell your healthcare provider about all of your medical conditions, including if you: • • o o • Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. | |
|
How should I use Alhemo? Read the Instructions for Use that comes with Alhemo for information about how to prepare and inject a dose of Alhemo, and how to properly throw away (dispose of) used Alhemo pens and needles. • • • • • • • • • • • • • • o o o o | |
|
What are the possible side effects of Alhemo? Alhemo may cause serious side effects, including: • | |
|
o |
o |
|
o |
o |
|
o |
o |
|
o |
o |
|
o | |
|
• | |
|
o |
o |
|
o |
o |
|
o |
o |
|
o |
o |
|
o | |
|
The most common side effects of Alhemo include: | |
|
• |
• • |
|
These are not all the possible side effects of Alhemo. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1‑800‑FDA‑1088. | |
|
How should I store Alhemo? • o • o o o • • • • • Keep Alhemo and all medicine out of the reach of children. | |
|
General information about the safe and effective use of Alhemo. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Alhemo for a condition for which it was not prescribed. Do not give Alhemo to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Alhemo that is written for health professionals. | |
|
What are the ingredients in Alhemo? Active ingredient: concizumab-mtci Inactive ingredients: arginine hydrochloride, histidine, phenol, polysorbate 80, sodium chloride, sucrose, and water for injection. Hydrochloric acid and sodium hydroxide may be added for pH adjustment. | |
|
Patent Information: http://novonordisk-us.com/products/product- patents.html Alhemo® is a registered trademark of Novo Nordisk Health Care AG. For information about Alhemo contact: Novo Nordisk Inc., 800 Scudders Mill Road Plainsboro, NJ 08536. Manufactured by: Novo Nordisk Inc., 800 Scudders Mill Road, Plainsboro, NJ 08536 U.S. License No. 1261 At: Novo Nordisk A/S, Novo Allé 1, 2880 Bagsværd, Denmark © 2025 Novo Nordisk For more information, go to Alhemo.com or call 1-844-668-6732. |
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised:07/2025
