Allopurinol
Allopurinol Tablets USP
f91c2183-e41d-45bc-87aa-edfca5eebac5
HUMAN PRESCRIPTION DRUG LABEL
Nov 28, 2023
Bryant Ranch Prepack
DUNS: 171714327
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
allopurinol
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
DESCRIPTION SECTION
DESCRIPTION
Allopurinol, USP has the following structural formula:
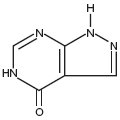
Allopurinol, USP is known chemically as 1, 5-dihydro-4 H-pyrazolo [3, 4- d] pyrimidin-4-one. It is a xanthine oxidase inhibitor which is administered orally. Each scored white tablet contains 100 mg allopurinol and the inactive ingredients colloidal silicon dioxide, lactose monohydrate, magnesium stearate, microcrystalline cellulose and sodium starch glycolate. Each scored orange tablet contains 300 mg allopurinol and the inactive ingredients colloidal silicon dioxide, FD&C Yellow No. 6 Lake, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and sodium starch glycolate. Soluble in solutions of potassium and sodium hydroxides, very slightly soluble in water and in alcohol; practically insoluble in chloroform and in ether.
