APur Thyroid
APur Thyroid™ (thyroid tablets, USP)
a912e127-c0a5-482c-b06c-b657c433c6d9
HUMAN PRESCRIPTION DRUG LABEL
Jan 5, 2024
Vitruvias Therapeutics
DUNS: 079200795
Products 5
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
THYROID
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
THYROID
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
THYROID
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
THYROID
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
THYROID
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Drug Labeling Information
DESCRIPTION SECTION
DESCRIPTION
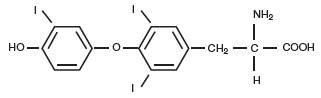
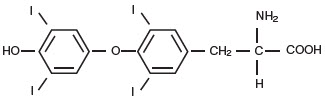
APur Thyroid™ (thyroid tablets, USP)1 for oral use is a natural preparation derived from porcine thyroid glands and may have a strong, characteristic odor. (T3 liothyronine is approximately four times as potent as T4 levothyroxine on a microgram for microgram basis.) They provide 38 mcg levothyroxine (T4) and 9 mcg liothyronine (T3) per grain of thyroid. The inactive ingredients are microcrystalline cellulose, lactose, silicon dioxide, and magnesium stearate.
|
STRUCTURAL FORMULAS | |
|
liothyronine (T3) |
Levothyroxine (T4) |
|
|
|
1
APur Thyroid™ (thyroid tablets, USP) has not been approved by FDA as a new drug.
WARNINGS SECTION
WARNINGS
|
Drugs with thyroid hormone activity, alone or together with other therapeutic agents, have been used for the treatment of obesity. In euthyroid patients, doses within the range of daily hormonal requirements are ineffective for weight reduction. Larger doses may produce serious or even life-threatening manifestations of toxicity, particularly when given in association with sympathomimetic amines such as those used for their anorectic effects. |
The use of thyroid hormones in the therapy of obesity, alone or combined with other drugs, is unjustified and has been shown to be ineffective. Neither is their use justified for the treatment of male or female infertility unless this condition is accompanied by hypothyroidism.
The active ingredient (desiccated natural thyroid) in APur Thyroid™ is derived from porcine (pig) thyroid glands of pigs processed for human food consumption. Vitruvias is not aware of any cases of disease transmission associated with the use of APur Thyroid™.
OVERDOSAGE SECTION
OVERDOSAGE
Signs and Symptoms
Excessive doses of thyroid result in a hypermetabolic state resembling in every respect the condition of endogenous origin. The condition may be self- induced.
Treatment of Overdosage
Dosage should be reduced or therapy temporarily discontinued if signs and symptoms of overdosage appear.
Treatment may be reinstituted at a lower dosage. In normal individuals, normal hypothalamic-pituitary-thyroid axis function is restored in 6 to 8 weeks after thyroid suppression.
Treatment of acute massive thyroid hormone overdosage is aimed at reducing gastrointestinal absorption of the drugs and counteracting central and peripheral effects, mainly those of increased sympathetic activity. Vomiting may be induced initially if further gastrointestinal absorption can reasonably be prevented and barring contraindications such as coma, convulsions, or loss of the gagging reflex. Treatment is symptomatic and supportive. Oxygen may be administered and ventilation maintained. Cardiac glycosides may be indicated if congestive heart failure develops. Measures to control fever, hypoglycemia, or fluid loss should be instituted if needed. Antiadrenergic agents, particularly propranolol, have been used advantageously in the treatment of increased sympathetic activity. Propranolol may be administered intravenously at a dosage of 1 to 3 mg, over a 10-minute period or orally, 80 to 160 mg/day, initially, especially when no contraindications exist for its use.
Other adjunctive measures may include administration of cholestyramine to interfere with thyroxine absorption, and glucocorticoids to inhibit conversion of T4 to T3.
HOW SUPPLIED SECTION
HOW SUPPLIED
APur Thyroid™ (thyroid tablets, USP) are supplied as follows: 15 mg (1/4 grain) are available in bottles of 100 (NDC 69680-149-00). 30 mg (1/2 grain) are available in bottles of 100 (NDC 69680-150-00). 60 mg (1 grain) are available in bottles of 100 (NDC 69680-151-00). 90 mg (1 & 1/2 grain) are available in bottles of 100 (NDC 69680-152-00). 120 mg (2 grain) are available in bottles of 100 (NDC 69680-153-00). The bottles of 100 have child-resistant closures.
APur Thyroid™ (thyroid tablets, USP) is an off-white to pale brown, round tablet, which may display flecks. The 15 mg and 30 mg tablets are flat-faced with a bevel edge, and the 60 mg, 90 mg, and 120 mg tablets have concave faces. One side is debossed with the letters "THY" and the other side with the strength code numbers as defined below.
|
Strength |
Code |
|---|---|
|
Note: (T3 liothyronine is approximately four times as potent as T4 levothyroxine on a microgram for microgram basis.) | |
|
¼ grain |
025 |
|
½ grain |
050 |
|
1 grain |
100 |
|
1 ½ grain |
150 |
|
2 grain |
200 |
Store in a tight container protected from light and moisture. Store between 15°C and 30°C (59°F and 86°F).