Trazodone Hydrochloride
These highlights do not include all the information needed to use TRAZODONE HYDROCHLORIDE TABLETS, USP safely and effectively. See full prescribing information for TRAZODONE HYDROCHLORIDE TABLETS, USP. TRAZODONE HYDROCHLORIDE tablets, USP for oral use Initial U.S. Approval: 1981
f7e6a78e-7ac3-35b9-e053-6294a90aabd0
HUMAN PRESCRIPTION DRUG LABEL
Aug 20, 2025
NuCare Pharmaceuticals,Inc.
DUNS: 010632300
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Trazodone Hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

DESCRIPTION SECTION
11 DESCRIPTION
Trazodone hydrochloride tablets for oral administration contain trazodone hydrochloride, a selective serotonin reuptake inhibitor and 5HT2 receptor antagonist. Trazodone hydrochloride is a triazolopyridine derivative designated as 2-[3-[4-(3-chlorophenyl)-1-piperazinyl]propyl]-1,2,4-triazolo[4,3-a]pyridin-3(2 H)-one hydrochloride. It is a white odorless crystalline powder which is freely soluble in water. The structural formula is represented as follows:
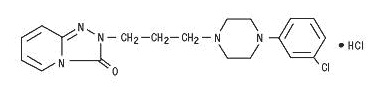
**Molecular Formula:**C 19H 22ClN 5O · HCl
**Molecular Weight:**408.33
Each tablet, for oral administration, contains 50 mg, 100 mg, 150 mg or 300 mg of trazodone hydrochloride, USP. In addition, each tablet contains the following inactive ingredients: colloidal silicon dioxide, magnesium stearate, microcrystalline cellulose, pregelatinized maize starch, sodium lauryl sulfate, and sodium starch glycolate.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Trazodone Hydrochloride Tablets, USP 100 mg are available for oral administration as white to off white, round, biconvex, uncoated tablets debossed with "13" bisect "31" on one side and plain on other side.
Bottles of 30, NDC 68071-2959-3
Bottles of 60 , NDC 68071-2959-6
-For 100 mg, break the score on either the left or right side of the tablet (two-thirds of a tablet).
Store at 20° to 25°C (68° to 77°F). Excursions permitted between 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Dispense with a child-resistant closure in a tight, light-resistant container as defined in the USP.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Suicidal Thoughts and Behaviors
Advise patients and caregivers to look for the emergence of suicidality, especially early during treatment and when the dosage is adjusted up or down and instruct them to report such symptoms to the healthcare provider [see Box Warning and Warnings and Precautions ( 5.1)] .
Dosage and Administration
Advise patients that trazodone hydrochloride tablets should be taken shortly after a meal or light snack. Advise patients regarding the importance of following dosage titration instructions [see Dosage and Administration ( 2)] .
Serotonin Syndrome
Caution patients about the risk of serotonin syndrome, particularly with the concomitant use of trazodone with other serotonergic drugs including triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, St. John's Wort, and with drugs that impair metabolism of serotonin (in particular, MAOIs, both those intended to treat psychiatric disorders and also others, such as linezolid). Patients should contact their health care provider or report to the emergency room if they experience signs or symptoms of serotonin syndrome [see Warnings and Precautions ( 5.2) and Drug Interactions ( 7)] .
Activation of Mania/Hypomania
Advise patients and their caregivers to observe for signs of activation of mania/hypomania and instruct them to report such symptoms to the healthcare provider [see Warnings and Precautions ( 5.7)] .
Increased Risk of Bleeding
Inform patients about the concomitant use of trazodone with aspirin, NSAIDs, other antiplatelet drugs, warfarin, or other anticoagulants because the combined use of drugs that interfere with serotonin reuptake and these medications has been associated with an increased risk of bleeding. Advise them to inform their health care providers if they are taking or planning to take any prescription or over-the-counter medications that increase the risk of bleeding [see Warnings and Precautions ( 5.5)] .
Discontinuation Syndrome
Advise patients not to abruptly discontinue trazodone hydrochloride tablets and to discuss any tapering regimen with their healthcare provider. Adverse reactions can occur when trazodone hydrochloride tablets is discontinued [see Warnings and Precautions ( 5.8)].
Concomitant Medications
Advise patients to inform their health care providers if they are taking, or plan to take any prescription or over-the-counter medications since there is a potential for interactions [see Drug Interactions ( 7.1)].
Pregnancy
Advise patients to notify their healthcare provider if they become pregnant or intend to become pregnant during therapy with trazodone hydrochloride tablets. Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to trazodone hydrochloride tablets during pregnancy [see Use in Special Populations ( 8.1)].

Manufactured by:
TORRENT PHARMACEUTICALS LTD., INDIA.
Manufactured for:
TORRENT PHARMA INC., Basking Ridge, NJ 07920
8090149 Revision: October 2022
SPL MEDGUIDE SECTION
MEDICATION GUIDE
Trazodone hydrochloride tablets, USP, for oral use
(traz' oh done hye" droe klor' ide)
What is the most important information I should know about trazodone hydrochloride tablets?
Antidepressant medicines, depression or other serious mental illnesses, and suicidal thoughts or actions: Talk to your healthcare provider about:
- All risks and benefits of treatment with antidepressant medicines
- All treatment choices for depression or other serious mental illnesses
**1.**Antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, and young adults within the first few months of treatment.
**2.**Depression and other serious mental illnesses are the most important causes of suicidal thoughts and actions.
Some people may have a higher risk of having suicidal thoughts or actions. These include people who have or have a family history of bipolar illness (also called manic-depressive illness) or suicidal thoughts or actions.
**3.**How can I watch for and try to prevent suicidal thoughts and actions?
- Pay close attention to any changes, especially sudden changes in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is started or when the dose is changed.
- Call your healthcare provider right away to report new or sudden changes in mood, behavior, thoughts or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled. Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
Call a healthcare provider right away if you have any of the following symptoms, especially if they are new, worse, or worry you:
- Thoughts about suicide or dying
- Attempts to commit suicide
- New or worse depression
- New or worse anxiety
- Feeling very agitated or restless
- Panic attacks
- Trouble sleeping (insomnia)
- New or worse irritability
- Acting aggressive, being angry or violent
- Acting on dangerous impulses
- An extreme increase in activity and talking (mania)
- Other unusual changes in behavior or mood
What else do I need to know about antidepressant medicines?
*Never stop an antidepressant medicine without first talking to a healthcare provider. Stopping an antidepressant medicine suddenly can cause other symptoms. *Antidepressants are medicines used to treat depression and other illnesses. It is important to discuss all the risks of treating depression and also the risks of not treating it. You should discuss all treatment choices with your healthcare provider, not just the use of antidepressants. *Antidepressant medicines have other side effects. Talk to your healthcare provider about the side effects of your medicines. *Antidepressant medicines can interact with other medicines. Know all of the medicines that you take. Keep a list of all medicines to show your healthcare provider. Do not start new medicines without first checking with your healthcare provider.
It is not known if trazodone is safe and effective in children.
What are trazodone hydrochloride tablets?
Trazodone hydrochloride tablets are a prescription medicine used in adults to treat major depressive disorder (MDD). Trazodone belongs to a class of medicines known as SSRIs (or selective serotonin reuptake inhibitors).
Do not take trazodone hydrochloride tablets**:**
- If you take a monoamine oxidase inhibitor (MAOI). Ask your healthcare provider or pharmacist if you are not sure if you take an MAOI, including the antibiotic linezolid, and intravenous methylene blue.
- Do not take an MAOI within 2 weeks of stopping trazodone hydrochloride tablets unless directed to do so by your healthcare provider.
- Do not start trazodone hydrochloride tablets if you stopped taking an MAOI in the last 2 weeks unless directed to do so by your healthcare provider.
Before you take trazodone hydrochloride tablets tell your healthcare provider about all of your medical conditions, including if you:
- have heart problems, including QT prolongation or a family history of it
- have ever had a heart attack
- have bipolar disorder
- have liver or kidney problems
- have other serious medical conditions
- are pregnant or plan to become pregnant. It is not known if trazodone hydrochloride tablets will harm your unborn baby. Talk to your healthcare provider about the risk to your unborn baby if you take trazodone hydrochloride tablets.
- If you become pregnant during treatment with trazodone hydrochloride tablets, talk to your healthcare provider about registering with the National Pregnancy Registry for Antidepressants. You can register by calling 1-844-405-6185.
- are breastfeeding or plan to breastfeed. Trazodone hydrochloride tablets passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you take trazodone hydrochloride tablets.
- have taken a Monoamine Oxidase Inhibitor (MAOI) or if you have stopped taking an MAOI in the last 2 weeks.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Using trazodone hydrochloride tablets with certain other medicines can affect each other causing serious side effects.
Especially tell your healthcare provider if you take:
- triptans used to treat migraine headache
- medicines used to treat mood, anxiety, psychotic or thought disorders, including tricyclics, lithium, SSRIs, SNRIs, buspirone, or antipsychotics
- tramadol
- over-the-counter supplements such as tryptophan or St. John's Wort
- nonsteroidal anti-inflammatory drugs (NSAIDS)
- aspirin
- warfarin (Coumadin, Jantoven)
- phenytoin (Mesantoin)
- diuretics
Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I take trazodone hydrochloride tablets?
- Take trazodone hydrochloride tablets exactly as your healthcare provider tells you.
- Trazodone hydrochloride tablets should be taken shortly after a meal or light snack.
- If you feel drowsy after taking trazodone hydrochloride tablets, talk to your healthcare provider. Your healthcare provider may change your dose or the time of day you take your trazodone hydrochloride tablets.
- Do not stop taking trazodone hydrochloride tablets without talking to your healthcare provider.
- Trazodone hydrochloride tablets should be swallowed whole or broken in half along the score line. Do not chew or crush trazodone hydrochloride tablets. Tell your healthcare provider if you cannot swallow trazodone either whole or as a half tablet.
- If you take too much trazodone hydrochloride, call your healthcare provider, your poison control center at 1-800-222-1222, or go to the nearest emergency room right away.
What should I avoid while taking trazodone hydrochloride tablets?
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how trazodone hydrochloride tablets affects you. Trazodone hydrochloride tablets can slow your thinking and motor skills.
- Do not drink alcohol or take other medicines that make you sleepy or dizzy while taking trazodone hydrochloride tablets until you talk with your healthcare provider. Trazodone hydrochloride tablets may make your sleepiness or dizziness worse if you take it with alcohol or other medicines that cause sleepiness or dizziness.
What are the possible side effects of trazodone hydrochloride tablets?
Trazodone hydrochloride tablets can cause serious side effects or death, including:
*See "What is the most important information I should know about trazodone hydrochloride tablets?" *Serotonin syndrome. Symptoms of serotonin syndrome include: agitation, hallucinations, problems with coordination, fast heartbeat, tight muscles, trouble walking, sweating, fever, nausea, vomiting, and diarrhea. *Irregular or fast heartbeat or faint (QT prolongation) *Low blood pressure. You feel dizzy or faint when you change positions (go from sitting to standing) *Unusual bruising or bleeding *Erection lasting for more than 6 hours (Priapism) *Feeling high or in a very good mood, then becoming irritable, or having too much energy, feeling like you have to keep talking or do not sleep (mania). *Withdrawal symptoms. Symptoms of withdrawal can include anxiety, agitation, and sleep problems. Do not stop taking trazodone hydrochloride tablets without talking to your healthcare provider. *Visual problems. * Eye pain * Changes in vision * Swelling or redness in or around the eye
Only some people are at risk for these problems. You may want to undergo an eye examination to see if you are at risk and receive preventative treatment if you are.
*Low sodium in your blood (hyponatremia). Symptoms of hyponatremia include: headache, feeling weak, feeling confused, trouble concentrating, memory problems and feeling unsteady when you walk.
Get medical help right away, if you have any of the symptoms listed above.
The most common side effects of trazodone hydrochloride tablets include:
- Swelling
- Blurred vision
- Dizziness
- Sleepiness
- Tiredness
- Diarrhea
- Stuffy nose
- Weight loss
These are not all the possible side effects of trazodone hydrochloride tablets. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800- FDA-1088.
How should I store trazodone hydrochloride tablets?
- Store trazodone hydrochloride tablets at room temperature between 68°F to 77°F (20° C to 25°C).
- Keep in tight container
- Keep out of the light
- Safely throw away medicine that is out of date or no longer needed.
Keep trazodone hydrochloride tablets and all medicines out of the reach of children.
General information about the safe and effective use of trazodone hydrochloride tablets.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use trazodone hydrochloride tablets for a condition for which it was not prescribed. Do not give trazodone hydrochloride tablets to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about trazodone hydrochloride tablets that is written for health professionals .
What are the ingredients in trazodone hydrochloride tablets?
**Active ingredient:**trazodone hydrochloride, USP
Inactive ingredients: colloidal silicon dioxide, magnesium stearate, microcrystalline cellulose, pregelatinized maize starch, sodium lauryl sulfate, and sodium starch glycolate.

Manufactured by:
TORRENT PHARMACEUTICALS LTD., INDIA.
Manufactured for:
TORRENT PHARMA INC., Basking Ridge, NJ 07920
For more information, go to www.torrentpharma.com or call 1-800-FDA-1088
This Medication Guide has been approved by the U.S. Food and Drug Administration.
8090150 Revised: October 2022
