Robinul
These highlights do not include all the information needed to use ROBINUL and ROBINUL FORTE safely and effectively. See full prescribing information for ROBINUL and ROBINUL FORTE. ROBINUL® and ROBINUL® FORTE (glycopyrrolate) tablets, for oral use Initial U.S. Approval: 1961
94fb029a-6371-4b1c-89de-35e8209c7cab
HUMAN PRESCRIPTION DRUG LABEL
Sep 22, 2022
Casper Pharma LLC
DUNS: 080025838
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Glycopyrrolate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Glycopyrrolate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Robinul**®****(glycopyrrolate, USP) tablets 1 mg**
Bottles of 90 NDC 70199-007-90

Robinul**®**** Forte (glycopyrrolate, USP) tablets 2mg**
** Bottles of 90 NDC 70199-008-90**
****
INDICATIONS & USAGE SECTION
1 INDICATIONS & USAGE
ROBINUL and ROBINUL FORTE are indicated in adults to reduce symptoms of a peptic ulcer as an adjunct to treatment of peptic ulcer.
Limitations of Use
ROBINUL and ROBINUL FORTE are not indicated as monotherapy for the treatment of peptic ulcer because effectiveness in peptic ulcer healing has not been established.
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
ROBINUL and ROBINUL FORTE are contraindicated in:
• Patients at risk for anticholinergic toxicity due to an underlying medical condition, including:
o Glaucoma [see Warnings and Precautions (5.1)]
o Obstructive uropathies, including prostatic hypertrophy
o Mechanical obstructive diseases of the gastrointestinal tract (e.g.,
pyloroduodenal stenosis, strictures) [see Warnings and Precautions (5.2)]
o Gastrointestinal motility disorders (e.g., achalasia, paralytic ileus,
intestinal atony) [see Warnings and Precautions (5.3)]
o Bleeding gastrointestinal ulcer
o Active inflammatory or infectious colitis which can lead to toxic megacolon
o History of or current toxic megacolon
o Myasthenia gravis
• Patients with a hypersensitivity to glycopyrrolate or any of the inactive ingredients in ROBINUL and ROBINUL FORTE [see Adverse Reactions (6) and Description (11)].
• Patients at risk for anticholinergic toxicity due to various underlying medical conditions. (4, 5.1, 5.2, 5.3)
• Hypersensitivity to glycopyrrolate or the inactive ingredients. (4)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following serious or otherwise important adverse reactions are discussed elsewhere in the labeling:
• Precipitation of Acute Glaucoma [see Warnings and Precautions (5.1)]
• Partial or Complete Mechanical Intestinal Obstruction [see Warnings and Precautions (5.2)]
• Gastrointestinal Adverse Reactions due to Decreased Gastrointestinal Motility [see Warnings and Precautions (5.3)]
• Cognitive and Visual Adverse Reactions [see Warnings and Precautions (5.4)]
• Heat Prostration at High Environmental Temperatures [see Warnings and Precautions (5.5)]
• Other Conditions Exacerbated by Anticholinergic Adverse Reactions [see Warnings and Precautions (5.6)]
• Increased Risk of Anticholinergic Adverse Reactions in Geriatric Patients [see Warnings and Precautions (5.7)]
The following adverse reactions associated with the use of glycopyrrolate, or other anticholinergic drugs, were identified in clinical studies or postmarketing reports. Because some of these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiac Disorders: chest, pain, hypertension, tachycardia
Endocrine Disorders: decreased sweating
Eye Disorders: blurred vision, cycloplegia, dilatation of the pupil, increased ocular tension
Gastrointestinal Disorders: bloated feeling, constipation, dry mouth, dysgeusia, nausea, vomiting
Immune System Disorders: anaphylaxis [see Contraindications (4)]
Nervous System Disorders: agitation, dizziness, drowsiness, headache, insomnia, mental confusion, nervousness, weakness
Respiratory Disorders: respiratory depression, throat irritation
Renal and Urinary Disorders: urinary hesitancy, urinary retention
Reproductive System and Breast Disorders: impotence, suppression of lactation
Vascular Disorders: flushing
Adverse reactions include blurred vision, drowsiness, decreased sweating, flushing, vomiting, constipation, dry mouth, tachycardia, and urinary retention. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Casper Pharma LLC at 1-844-5-CASPER (1-844-522-7737) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Other Anticholinergic Drugs
There is potential for an additive interaction between glycopyrrolate and concomitantly used anticholinergic drugs (e.g., tricyclic antidepressants, anti-epileptics, class I antiarrhythmics, antispasmodics, amantadine) resulting in increased anticholinergic adverse reactions. Co-administration of antipsychotics with glycopyrrolate may lead to worsening of tardive dyskinesia. ROBINUL and ROBINUL FORTE are not recommended in patients taking other anticholinergic drugs [see Warnings and Precautions (5.3, 5.4, 5.6)].
7.2 Drugs with Altered Absorption due to Decreased Gastrointestinal
Motility and Increased Transit Time
Decreased gastrointestinal motility by glycopyrrolate may impact absorption of other drugs leading to increased or decreased drug exposure. ROBINUL and ROBINUL FORTE are not recommended in patients taking other drugs that are affected by altered gastrointestinal motility [see Warnings and Precautions (5.3)].
7.3 Gastrointestinal Toxicity with Solid Oral Dosage Forms of Potassium
Chloride
Oral glycopyrrolate may worsen gastrointestinal mucosal injury reported with solid oral dosage forms of potassium chloride due to decreased gastric motility and increased transit time, leading to prolonged contact with the gastrointestinal mucosa. ROBINUL and ROBINUL FORTE are not recommended in patients taking solid oral dosage forms of potassium chloride.
• Other Anticholinergic Drugs: Concomitant use is not recommended. (5.3, 5.4, 5.6, 7.1)
• Drugs with Altered Absorption due to Decreased GI Motility: Concomitant use is not recommended. (7.2)
• GI Toxicity with Solid Oral Dosage Forms of Potassium Chloride: Concomitant use is not recommended. (7.3)
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE & ADMINISTRATION
2.1 Important Dosing Information
• ROBINUL FORTE is not recommended for patients in whom a lower dosage strength of oral glycopyrrolate (e.g., ROBINUL or another 1 mg tablet strength) is appropriate for initial or maintenance treatment because the dosage strength of ROBINUL FORTE may exceed the recommended initial and maintenance dosage of oral glycopyrrolate tablets.
2.2 Recommended Dosage
• The recommended initial dosage of ROBINUL for adults is 1 mg three times daily (in the morning, early afternoon, and at bedtime). Some patients may require 2 mg at bedtime to assure overnight control of symptoms. For maintenance, a dosage of 1 mg twice a day is frequently adequate.
• The recommended dosage of ROBINUL FORTE for adults is 2 mg two or three times daily at equally spaced intervals.
• The maximum recommended daily dosage of glycopyrrolate is 8 mg.
• Use the lowest effective dosage of glycopyrrolate to control symptoms. If patients can be titrated to a lower dose, switch from ROBINUL FORTE to ROBINUL or another 1 mg oral tablet of glycopyrrolate.
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS & STRENGTHS
Tablets:
• 1 mg, white, round, flat-faced, beveled-edge tablet, debossed “CS” and “007”
on one side and functionally scored on the other side.
• 2 mg, white, round, flat-faced, beveled-edge tablet, debossed “CS” score
“008” on one side and plain on the other side.
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Over decades of use, there is an absence of published data on orally administered glycopyrrolate in pregnant women, including an absence of any reports of a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. In animal studies, at non-maternally toxic doses of oral glycopyrrolate, there were no adverse developmental effects in rats or rabbits. A pre- and post-natal development study of oral glycopyrrolate in rats showed a decrease in pup mean bodyweight that recovered post nursing, with no other developmental effects observed (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
At non-maternally toxic doses of oral glycopyrrolate, there were no effects on embryo-fetal development or toxicity in rats or rabbits. A pre- and post-natal development study of oral glycopyrrolate in rats showed a decrease in pup mean bodyweight that recovered post nursing, with no other developmental effects observed.
In a published reproductive and developmental study, male and female rats were administered glycopyrrolate in the diet at 0 mg/kg/day, 32.5 mg/kg/day, 63 mg/kg/day, and 130 mg/kg/day for 3 weeks to 5 weeks and through up to three consecutive litters. There was no indication of abnormalities in the pups of treated dams. There was a decreased rate of conception and in survival rate at weaning for all treated animals in a dose-related manner. Diminished rates of conception may be due to diminished seminal secretion [see Nonclinical Toxicology (13.1)].
8.2 Lactation
Risk Summary
There are no data on the presence of glycopyrrolate in either human or animal milk, the effects on the breastfed infant, or the effects on milk production. As with other anticholinergic drugs, glycopyrrolate may cause suppression of lactation. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for ROBINUL and ROBINUL FORTE and any potential adverse effects on the breastfed infant from ROBINUL and ROBINUL FORTE.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
8.5 Geriatric Use
Geriatric patients 65 years of age and older may be more sensitive to the anticholinergic adverse reactions of glycopyrrolate leading to complications of urinary retention, bowel obstruction, heat prostration, arrhythmias, delirium, and falls or fractures; therefore, ROBINUL and ROBINUL FORTE are not recommended in geriatric patients and may be contraindicated in some geriatric patients with underlying medical conditions [see Contraindications (4) and Warnings and Precautions (5)].
8.6 Renal Impairment
Glycopyrrolate is substantially excreted by the kidney [see Clinical Pharmacology (12.3)].
Monitor patients with renal impairment for anticholinergic adverse reactions [see Adverse Reactions (6)]. If anticholinergic adverse reactions occur, discontinue ROBINUL and ROBINUL FORTE.
• Renal Impairment: Monitor patients with renal impairment; if anticholinergic adverse reactions occur, discontinue use. (8.6)
OVERDOSAGE SECTION
10 OVERDOSAGE
Signs and symptoms of glycopyrrolate overdosage are related to excessive anti- muscarinic anticholinergic activity and are generally peripheral (e.g., flushing, hyperthermia, tachycardia, ileus, urinary retention, loss of ocular accommodation, and light sensitivity due to mydriasis), but central nervous system toxicity (agitation, seizures, hyperthermia) may also occur.
If over-exposure occurs, call the Poison Control Center at 1-800-222-1222 for current information on the management of glycopyrrolate poisoning and overdosage.
Management of glycopyrrolate overdosage is based upon presenting signs and symptoms, including close observation for severe or life-threatening complications which may require respiratory and cardiovascular monitoring and support. Consider administration of activated charcoal and/or use of a reversible anticholinesterase as appropriate or recommended by Poison Control.
DESCRIPTION SECTION
11 DESCRIPTION
ROBINUL and ROBINUL FORTE tablets contain synthetic anticholinergic glycopyrrolate. Glycopyrrolate is a quaternary ammonium compound with the following chemical name: 3-[(cyclopentyl hydroxyphenylacetyl)oxy]-1,1-dimethylpyrrolidinium bromide. The molecular formula for glycopyrrolate is C19H28BrNO3, the molecular weight is 398.3 g/mol, and the structural formula is:
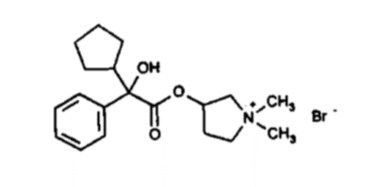
Each ROBINUL tablet contains glycopyrrolate, USP 1 mg, as the active ingredient. Each ROBINUL FORTE tablet contains glycopyrrolate, USP 2 mg, as the active ingredient. The inactive ingredients are dibasic calcium phosphate, lactose monohydrate, magnesium stearate, povidone, and sodium starch glycolate.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Glycopyrrolate, an anticholinergic (antimuscarinic) agent, inhibits the action of acetylcholine on parietal cells in the stomach and decreases the volume and acidity of gastric secretions.
12.2 Pharmacodynamics
No formal pharmacodynamic studies have been conducted with ROBINUL and ROBINUL FORTE.
12.3 Pharmacokinetics
Patients with Renal Impairment
In the published literature, glycopyrrolate 4 mcg/kg was administered intravenously (ROBINUL and ROBINUL FORTE are not recommended for intravenous use) in uremic patients undergoing renal transplantation surgery. The mean AUC (10.6 mcg·h/L) and 24-hour urinary excretion (7%) for glycopyrrolate were significantly different from normal healthy adult subjects undergoing general surgery (3.7 mcg·h/L, and 65%, respectively) [see Use in Specific Populations (8.6)].
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility
Reproduction studies in rats resulted in diminished rates of conception in a dose-related manner. Studies in dogs suggest that diminished rates of conception may be due to diminished seminal secretion, which is evident at high doses of glycopyrrolate.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
ROBINUL (glycopyrrolate) tablets, 1 mg are white, round, flat-faced, beveled- edge tablet, debossed “CS” and “007” on one side and functionally scored on the other side. Available as
• Bottles of 30 NDC 70199-007-30
• Bottles of 90 NDC 70199-007-90
ROBINUL FORTE (glycopyrrolate) tablets, 2 mg are white, round, flat-faced, beveled-edge tablet, debossed “CS” score “008” on one side and plain on the other side. Available as
• Bottles of 30 NDC 70199-008-30
• Bottles of 90 NDC 70199-008-90
Store at controlled room temperature, 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Keep out of reach of children.
Dispense in a tight container.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Precipitation of Acute Glaucoma
Advise patients to discontinue ROBINUL and ROBINUL FORTE and promptly seek medical care if they experience symptoms of acute angle-closure glaucoma (pain and reddening of the eyes accompanied by dilated pupils) [see Warnings and Precautions (5.1)].
Partial or Complete Mechanical Intestinal Obstruction
Advise patients to contact their healthcare provider if diarrhea occurs, especially in patients with ileostomy or colostomy [see Warnings and Precautions (5.2)].
Gastrointestinal Adverse Reactions Due to Decreased Gastrointestinal Motility
Inform patients that ROBINUL and ROBINUL FORTE may cause adverse reactions related to decreased gastrointestinal motility and report to their healthcare provider if they experience symptoms such as vomiting, early satiety, abdominal distention, and constipation [see Warnings and Precautions (5.3)].
Cognitive and Visual Adverse Reactions
Inform patients that ROBINUL and ROBINUL FORTE may cause cognitive or visual impairment and not operate motor vehicles or other dangerous machinery or perform other hazardous tasks until they are reasonably certain that ROBINUL and ROBINUL FORTE do not affect them adversely. Advise patients to discontinue ROBINUL and ROBINUL FORTE immediately and contact their healthcare provider if symptoms develop (e.g., drowsiness or blurred vision) [see Warnings and Precautions (5.4)].
Heat Prostration at High Environmental Temperatures
Inform patients that ROBINUL and ROBINUL FORTE can reduce sweating, leading to the possibility of heat exhaustion or heat stroke. Advise patients to avoid exposure to hot or very warm environmental temperatures [see Warnings and Precautions (5.5)].
Manufactured for:
Casper Pharma LLC
East Brunswick, NJ 08816
Manufactured by:
Suven Pharmaceuticals Limited,
Telangana, India
M.L.No.: 24/MD/AP/2009/F/CC
Revised: 09/2022
PIB00890-05
ROBINUL® and ROBINUL FORTE® are registered trademarks of CASPER PHARMA LLC.
