fesoterodine fumarate
These highlights do not include all the information needed to use FESOTERODINE FUMARATE EXTENDED-RELEASE TABLETS safely and effectively. See full prescribing information for FESOTERODINE FUMARATE EXTENDED-RELEASE TABLETS. FESOTERODINE fumarate extended-release tablets, for oral useInitial U.S. Approval: 2008
2566f5f2-3256-4f0d-818a-7f34196dbb31
HUMAN PRESCRIPTION DRUG LABEL
Jan 31, 2023
Lupin Pharmaceuticals, Inc.
DUNS: 089153071
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
fesoterodine fumarate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
fesoterodine fumarate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
Fesoterodine fumarate extended-release tablets are contraindicated in patients with any of the following:
- known or suspected hypersensitivity to fesoterodine fumarate or any of its ingredients, or to tolterodine tartrate tablets or tolterodine tartrate extended-release capsules [see Clinical Pharmacology (12.1)]. Reactions have included angioedema [see Warnings and Precautions (5.1)].
- urinary retention [see Warnings and Precautions (5.2)]
- gastric retention [see Warnings and Precautions (5.3)]
- uncontrolled narrow-angle glaucoma [see Warnings and Precautions (5.4)]
- Known or suspected hypersensitivity to fesoterodine fumarate or any of its ingredients or to tolterodine tartrate tablets or tolterodine tartrate extended-release capsules. (4)
- Urinary retention (4)
- Gastric retention (4)
- Uncontrolled narrow-angle glaucoma. (4)
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage for Adult Patients with OAB
The recommended starting dosage of fesoterodine fumarate in adults is 4 mg orally once daily. Based upon individual response and tolerability, increase to the maximum dosage of fesoterodine fumarate 8 mg once daily. For administration instructions, see Dosage and Administration (2.6).
Pediatric use information is approved for Pfizer Inc.'s TOVIAZ® (fesoterodine fumarate) extended-release tablets. However, due to Pfizer Inc.'s marketing exclusivity rights, this drug product is not labeled with that information.
2.3 Recommended Dosage in Adult Patients with Renal Impairment
The recommended dosage of fesoterodine fumarate in adult patients with renal impairment is described in Table 1 [see Use in Specific Populations (8.6)]. For administration instructions, see Dosage and Administration (2.6).
Table 1: Fesoterodine fumarate Recommended Dose in Adult Patients with Renal Impairment (Administered Orally Once Daily)
| |
|
** Estimated Creatinine Clearance******* |
** Recommended Dose** |
|
CLcr 30 to 89 mL/min |
8 mg |
|
CLcr 15 to 29 mL/min |
4 mg |
|
CLcr <15 mL/min |
4 mg |
Pediatric use information is approved for Pfizer Inc.'s TOVIAZ® (fesoterodine fumarate) extended-release tablets. However, due to Pfizer Inc.'s marketing exclusivity rights, this drug product is not labeled with that information.
2.5 Fesoterodine fumarate Dosage Modifications Due to Strong CYP3A4
Inhibitors
Adult Patients with OAB
The maximum recommended dosage is fesoterodine fumarate 4 mg orally once daily in adult patients taking strong CYP3A4 inhibitors [see Drug Interactions (7.2) and Clinical Pharmacology (12.3)]. For administration instructions, see Dosage and Administration (2.6).
Pediatric use information is approved for Pfizer Inc.'s TOVIAZ® (fesoterodine fumarate) extended-release tablets. However, due to Pfizer Inc.'s marketing exclusivity rights, this drug product is not labeled with that information.
2.6 Administration Instructions
Swallow fesoterodine fumarate whole with liquid. Do not chew, divide, or crush. Take with or without food [see Clinical Pharmacology (12.3)].
- OAB in Adults : The recommended starting dosage is 4 mg orally once daily. Based upon individual response and tolerability, increase to the maximum dosage of 8 mg once daily. (2.1)
- Adult Patients with Renal or Hepatic Impairment: Refer to the full prescribing information for recommended dosage. (2.3)
- Dosage Modifications Due to Strong CYP3A4 Inhibitors : Refer to the full prescribing information for recommended dosage. (2.5)
- Administration : Swallow whole with liquid. Do not chew, divide, or crush. Take with or without food. (2.6)
DESCRIPTION SECTION
11 DESCRIPTION
Fesoterodine fumarate extended-release tablet contains fesoterodine fumarate and is an extended-release tablet. Fesoterodine is rapidly de-esterified to its active metabolite (R)-2-(3-diisopropylamino-1-phenylpropyl)-4-hydroxymethyl-phenol, or 5-hydroxymethyl tolterodine, which is a muscarinic receptor antagonist.
Chemically, fesoterodine fumarate is designated as (R-(+)-2-(-3-Diisopropylamino-1-phenylpropyl)-4-hydroxymethyl- phenylisobutyrate ester hydrogen fumarate. The empirical formula is C30H41NO7 and its molecular weight is 527.66. The structural formula is:
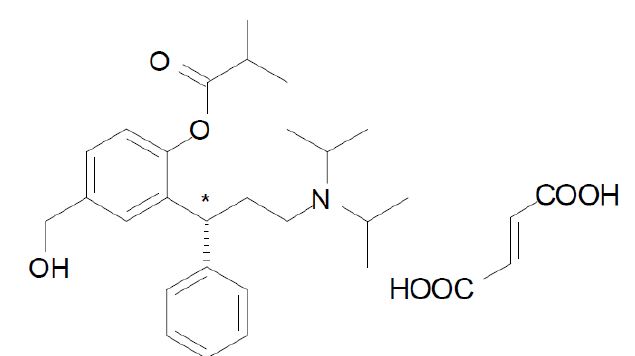
The asterisk (*) indicates the chiral carbon.
Fesoterodine fumarate is a white to off-white powder, which is very slightly soluble in water. Each fesoterodine fumarate extended-release tablets contains either 4 mg or 8 mg of fesoterodine fumarate and the following inactive ingredients: FD&C Blue #2 aluminium lake, hypromellose, lactose monohydrate, magnesium stearate, polyethylene glycol, polyvinyl alcohol, soya lecithin, talc, titanium dioxide and xylitol.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity
No evidence of drug-related carcinogenicity was found in 24-month studies with oral administration to mice and rats. The highest tolerated doses in mice (females 45 to 60 mg/kg/day, males 30 to 45 mg/kg/day) correspond to 11 to 19 times (females) and 4 to 9 times (males) the estimated human AUC values reached with fesoterodine 8 mg, which is the Maximum Recommended Human Dose (MRHD). In rats, the highest tolerated dose (45 to 60 mg/kg/day) corresponds to 3 to 8 times (females) and 3 to 14 times (males) the estimated human AUC at the MRHD.
Mutagenesis
Fesoterodine was not mutagenic or genotoxic in vitro (Ames tests, chromosome aberration tests) or in vivo (mouse micronucleus test).
Impairment of Fertility
Fesoterodine had no effect on male reproductive function or fertility at doses up to 45 mg/kg/day in mice. At 45 mg/kg/day, a lower number of corpora lutea, implantation sites and viable fetuses was observed in female mice administered fesoterodine for 2-weeks prior to mating and continuing through day 7 of gestation. The maternal No-Observed-Effect Level (NOEL) and the NOEL for effects on reproduction and early embryonic development were both 15 mg/kg/day. At the NOEL, the systemic exposure, based on AUC, was 0.6 to 1.5 times higher in mice than in humans at the MRHD, whereas based on peak plasma concentrations, the exposure in mice was 5 to 9 times higher.
