Registrants1
Companies and organizations registered with the FDA for this drug approval, including their contact information and regulatory details.
659220961
Manufacturing Establishments1
FDA-registered manufacturing facilities and establishments involved in the production, packaging, or distribution of this drug product.
Strides Pharma Science Limited
Strides Pharma Global Pte. Ltd.
118344504
Products4
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Olanzapine
Product Details
Olanzapine
Product Details
Olanzapine
Product Details
Olanzapine
Product Details
Drug Labeling Information
Complete FDA-approved labeling information including indications, dosage, warnings, contraindications, and other essential prescribing details.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
DESCRIPTION SECTION
11 DESCRIPTION
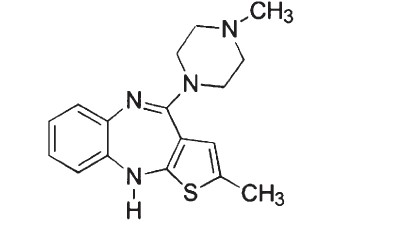
Olanzapine is an atypical antipsychotic that belongs to the thienobenzodiazepine class. The chemical designation is 2-methyl-4-(4-methyl-1-piperazinyl)-10H-thieno[2,3-b] [1,5]benzodiazepine. The molecular formula is C17H20N4S, which corresponds to a molecular weight of 312.44. The chemical structure is:
Olanzapine is a yellow crystalline solid, which is practically insoluble in water.
Olanzapine orally disintegrating tablets, USP are intended for oral administration only.
Each orally disintegrating tablet contains olanzapine equivalent to 5 mg (16 μmol), 10 mg (32 μmol), 15 mg (48 μmol) or 20 mg (64 μmol). It begins disintegrating in the mouth within seconds, allowing its contents to be subsequently swallowed with or without liquid. Olanzapine orally disintegrating tablets, USP also contains the following inactive ingredients: aspartame, crospovidone, magnesium stearate, mannitol, silicone dioxide, sorbitol, talc, and artificial pineapple flavor.
Meets USP Disintegration Test 2
INDICATIONS & USAGE SECTION
Highlight: Olanzapine is an atypical antipsychotic indicated: (1)
As oral formulation for the: (1)
-
Treatment of schizophrenia.(1.1)
- Adults: Efficacy was established in three clinical trials in patients with schizophrenia: two 6-week trials and one maintenance trial.(14.1)
- Adolescents (ages 13 to 17): Efficacy was established in one 6-week trial in patients with schizophrenia**(14.1)** . The increased potential (in adolescents compared with adults) for weight gain and dyslipidemia may lead clinicians to consider prescribing other drugs first in adolescents.(1.1)
-
Acute treatment of manic or mixed episodes associated with bipolar I disorder and maintenance treatment of bipolar I disorder.(1.2)
- Adults: Efficacy was established in three clinical trials in patients with manic or mixed episodes of bipolar I disorder: two 3- to 4-week trials and one maintenance trial.(14.2)
- Adolescents (ages 13 to 17): Efficacy was established in one 3-week trial in patients with manic or mixed episodes associated with bipolar I disorder**(14.2)** . The increased potential (in adolescents compared with adults) for weight gain and dyslipidemia may lead clinicians to consider prescribing other drugs first in adolescents.(1.2)
-
Medication therapy for pediatric patients with schizophrenia or bipolar I disorder should be under- taken only after a thorough diagnostic evaluation and with careful consideration of the potential risks.(1.3)
-
Adjunct to valproate or lithium in the treatment of manic or mixed episodes associated with bipolar I disorder.(1.2)
- Efficacy was established in two 6-week clinical trials in adults**(14.2)** . Maintenance efficacy has not been systematically evaluated.
As Olanzapine and Fluoxetine in Combination for the: (1)
-
Treatment of depressive episodes associated with bipolar I disorder.(1.5)
- Efficacy was established with Symbyax (olanzapine and fluoxetine in combination); refer to the product label for Symbyax.
-
Treatment of treatment resistant depression.(1.6)
- Efficacy was established with Symbyax (olanzapine and fluoxetine in combination) in adults; refer to the product label for Symbyax.
1 INDICATIONS AND USAGE
1.1 Schizophrenia
Oral olanzapine is indicated for the treatment of schizophrenia. Efficacy was established in three clinical trials in adult patients with schizophrenia: two 6-week trials and one maintenance trial. In adolescent patients with schizophrenia (ages 13 to 17), efficacy was established in one 6-week trial [seeClinical Studies (14.1)]****.
When deciding among the alternative treatments available for adolescents, clinicians should consider the increased potential (in adolescents as compared with adults) for weight gain and dyslipidemia. Clinicians should consider the potential long-term risks when prescribing to adolescents, and in many cases this may lead them to consider prescribing other drugs first in adolescents [seeWarnings and Precautions**(5.5)].**
1.2 Bipolar I Disorder (Manic or Mixed Episodes)
Monotherapy — Oral olanzapine is indicated for the acute treatment of manic or mixed episodes associated with bipolar I disorder and maintenance treatment of bipolar I disorder. Efficacy was established in three clinical trials in adult patients with manic or mixed episodes of bipolar I disorder: two 3- to 4-week trials and one monotherapy maintenance trial. In adolescent patients with manic or mixed episodes associated with bipolar I disorder (ages 13 to 17), efficacy was established in one 3-week trial [seeClinical Studies (14.2)].
When deciding among the alternative treatments available for adolescents, clinicians should consider the increased potential (in adolescents as compared with adults) for weight gain and dyslipidemia. Clinicians should consider the potential long-term risks when prescribing to adolescents, and in many cases this may lead them to consider prescribing other drugs first in adolescents [seeWarnings and Precautions (5.5)].
Adjunctive Therapy to Lithium or Valproate — Oral olanzapine is indicated for the treatment of manic or mixed episodes associated with bipolar I disorder as an adjunct to lithium or valproate. Efficacy was established in two 6-week clinical trials in adults. The effectiveness of adjunctive therapy for longer- term use has not been systematically evaluated in controlled trials [see Clinical Studies (14.2)].
1.3 Special Considerations in Treating Pediatric Schizophrenia and Bipolar
I Disorder
Pediatric schizophrenia and bipolar I disorder are serious mental disorders; however, diagnosis can be challenging. For pediatric schizophrenia, symptom profiles can be variable, and for bipolar I disorder, pediatric patients may have variable patterns of periodicity of manic or mixed symptoms. It is recommended that medication therapy for pediatric schizophrenia and bipolar I disorder be initiated only after a thorough diagnostic evaluation has been performed and careful consideration given to the risks associated with medication treatment. Medication treatment for both pediatric schizophrenia and bipolar I disorder should be part of a total treatment program that often includes psychological, educational and social interventions.
1.5 Olanzapine and Fluoxetine in Combination: Depressive Episodes
Associated with Bipolar I Disorder
Oral olanzapine and fluoxetine in combination is indicated for the treatment of depressive episodes associated with bipolar I disorder, based on clinical studies. When using olanzapine and fluoxetine in combination, refer to the Clinical Studies section of the package insert for Symbyax. Olanzapine monotherapy is not indicated for the treatment of depressive episodes associated with bipolar I disorder.
1.6 Olanzapine and Fluoxetine in Combination: Treatment Resistant
Depression
Oral olanzapine and fluoxetine in combination is indicated for the treatment of treatment resistant depression (major depressive disorder in patients who do not respond to 2 separate trials of different antidepressants of adequate dose and duration in the current episode), based on clinical studies in adult patients. When using olanzapine and fluoxetine in combination, refer to the Clinical Studies section of the package insert for Symbyax.
Olanzapine monotherapy is not indicated for the treatment of treatment resistant depression.
DOSAGE & ADMINISTRATION SECTION
Highlight:
Schizophrenia in adults (** 2.1** )
Oral: Start at 5 to 10 mg once daily; Target: 10 mg/day within several days
Schizophrenia in adolescents** (2.1)**
Oral: Start at 2.5 to 5 mg once daily; Target: 10 mg/day
Bipolar I Disorder (manic or mixed episodes) in adults (** 2.2** )
Oral: Start at 10 or 15 mg once daily
Bipolar I Disorder (manic or mixed episodes) in adolescents** (2.2)**
Oral: Start at 2.5 to 5 mg once daily; Target: 10 mg/day
Bipolar I Disorder (manic or mixed episodes) with lithium or valproate in adults (** 2.2** )
Oral: Start at 10 mg once daily
Depressive Episodes associated with Bipolar I Disorder in adults** (2.5)**
Oral in combination with fluoxetine: Start at 5 mg of oral olanzapine and 20 mg of fluoxetine once daily
Depressive Episodes associated with Bipolar I Disorder in children and adolescents** (2.5)**
Oral in combination with fluoxetine: Start at 2.5 mg of oral olanzapine and 20 mg of fluoxetine once daily
Treatment Resistant Depression in adults** (2.6)**
Oral in combination with fluoxetine: Start at 5 mg of oral olanzapine and 20 mg of fluoxetine once daily
- Lower starting dose recommended in debilitated or pharmacodynamically sensitive patients or patients with predisposition to hypotensive reactions, or with potential for slowed metabolism.(2.1)
- Olanzapine may be given without regard to meals.(2.1)
Olanzapine and Fluoxetine in Combination: (2)
- Dosage adjustments, if indicated, should be made with the individual components according to efficacy and tolerability.(2.5, 2.6)
- Olanzapine monotherapy is not indicated for the treatment of depressive episodes associated with bipolar I disorder or treatment resistant depression.(2.5, 2.6)
- Safety of coadministration of doses above 18 mg olanzapine with 75 mg fluoxetine has not been evaluated in adults.(2.5, 2.6)
- Safety of coadministration of doses above 12 mg olanzapine with 50 mg fluoxetine has not been evaluated in children and adolescents ages 10 to 17.(2.5)
2 DOSAGE AND ADMINISTRATION
2.1 Schizophrenia
Adults
Dose Selection — Oral olanzapine should be administered on a once-a-day schedule without regard to meals, generally beginning with 5 to 10 mg initially, with a target dose of 10 mg/day within several days. Further dosage adjustments, if indicated, should generally occur at intervals of not less than 1 week, since steady state for olanzapine would not be achieved for approximately 1 week in the typical patient. When dosage adjustments are necessary, dose increments/decrements of 5 mg QD are recommended.
Efficacy in schizophrenia was demonstrated in a dose range of 10 to 15 mg/day in clinical trials. However, doses above 10 mg/day were not demonstrated to be more efficacious than the 10 mg/day dose. An increase to a dose greater than the target dose of 10 mg/day (i.e., to a dose of 15 mg/day or greater) is recommended only after clinical assessment. Olanzapine is not indicated for use in doses above 20 mg/day.
Dosing in Special Populations — The recommended starting dose is 5 mg in patients who are debilitated, who have a predisposition to hypotensive reactions, who otherwise exhibit a combination of factors that may result in slower metabolism of olanzapine (e.g., nonsmoking female patients ≥65 years of age), or who may be more pharmacodynamically sensitive to olanzapine [see Warnings and Precautions (5.14),Drug Interactions (7), and Clinical Pharmacology(12.3)]. When indicated, dose escalation should be performed with caution in these patients.
Maintenance Treatment — The effectiveness of oral olanzapine, 10 mg/day to 20 mg/day, in maintaining treatment response in schizophrenic patients who had been stable on olanzapine for approximately 8 weeks and were then followed for relapse has been demonstrated in a placebo-controlled trial [seeClinical Studie****s (14.1)]. The healthcare provider who elects to use olanzapine for extended periods should periodically reevaluate the long-term usefulness of the drug for the individual patient.
Adolescents
Dose Selection — Oral olanzapine should be administered on a once-a-day schedule without regard to meals with a recommended starting dose of 2.5 or 5 mg, with a target dose of 10 mg/day. Efficacy in adolescents with schizophrenia was demonstrated based on a flexible dose range of 2.5 to 20 mg/day in clinical trials, with a mean modal dose of 12.5 mg/day (mean dose of 11.1 mg/day). When dosage adjustments are necessary, dose increments/decrements of 2.5 or 5 mg are recommended.
The safety and effectiveness of doses above 20 mg/day have not been evaluated in clinical trials [seeClinical Studies (14.1)].
Maintenance Treatment — The efficacy of olanzapine for the maintenance treatment of schizophrenia in the adolescent population has not been systematically evaluated; however, maintenance efficacy can be extrapolated from adult data along with comparisons of olanzapine pharmacokinetic parameters in adult and adolescent patients. Thus, it is generally recommended that responding patients be continued beyond the acute response, but at the lowest dose needed to maintain remission. Patients should be periodically reassessed to determine the need for maintenance treatment.
2.2 Bipolar I Disorder (Manic or Mixed Episodes)
Adults
Dose Selection for Monotherapy — Oral olanzapine should be administered on a once-a-day schedule without regard to meals, generally beginning with 10 or 15 mg. Dosage adjustments, if indicated, should generally occur at intervals of not less than 24 hours, reflecting the procedures in the placebo-controlled trials. When dosage adjustments are necessary, dose increments/decrements of 5 mg QD are recommended.
Short-term (3 to 4 weeks) antimanic efficacy was demonstrated in a dose range of 5 mg to 20 mg/day in clinical trials. The safety of doses above 20 mg/day has not been evaluated in clinical trials [seeClinical Studies (14.2)].
Maintenance Monotherapy — The benefit of maintaining bipolar I patients on monotherapy with oral olanzapine at a dose of 5 to 20 mg/day, after achieving a responder status for an average duration of 2 weeks, was demonstrated in a controlled trial [seeClinical Studies (14.2)]. The healthcare provider who elects to use olanzapine for extended periods should periodically reevaluate the long-term usefulness of the drug for the individual patient.
Dose Selection for Adjunctive Treatment — When administered as adjunctive treatment to lithium or valproate, oral olanzapine dosing should generally begin with 10 mg once-a-day without regard to meals.
Antimanic efficacy was demonstrated in a dose range of 5 mg to 20 mg/day in clinical trials [seeClinical Studies (14.2)]. The safety of doses above 20 mg/day has not been evaluated in clinical trials.
Adolescents
Dose Selection — Oral olanzapine should be administered on a once-a-day schedule without regard to meals with a recommended starting dose of 2.5 or 5 mg, with a target dose of 10 mg/day. Efficacy in adolescents with bipolar I disorder (manic or mixed episodes) was demonstrated based on a flexible dose range of 2.5 to 20 mg/day in clinical trials, with a mean modal dose of 10.7 mg/day (mean dose of 8.9 mg/day). When dosage adjustments are necessary, dose increments/decrements of 2.5 or 5 mg are recommended.
The safety and effectiveness of doses above 20 mg/day have not been evaluated in clinical trials [seeClinical Studies(14.2)].
Maintenance Treatment — The efficacy of olanzapine for the maintenance treatment of bipolar I disorder in the adolescent population has not been evaluated; however, maintenance efficacy can be extrapolated from adult data along with comparisons of olanzapine pharmacokinetic parameters in adult and adolescent patients. Thus, it is generally recommended that responding patients be continued beyond the acute response, but at the lowest dose needed to maintain remission. Patients should be periodically reassessed to determine the need for maintenance treatment.
2.3 Administration of Olanzapine Orally Disintegrating Tablets
Peel back foil on blister. Do not push tablet through foil. Immediately upon opening the blister, using dry hands, remove tablet and place entire olanzapine orally disintegrating tablet in the mouth. Tablet disintegration occurs rapidly in saliva so it can be easily swallowed with or without liquid.
2.5 Olanzapine and Fluoxetine in Combination: Depressive Episodes
Associated with Bipolar I Disorder
When using olanzapine and fluoxetine in combination, also refer to the Clinical Studies section of the package insert for Symbyax.
Adults
Oral olanzapine should be administered in combination with fluoxetine once daily in the evening, without regard to meals, generally beginning with 5 mg of oral olanzapine and 20 mg of fluoxetine. Dosage adjustments, if indicated, can be made according to efficacy and tolerability within dose ranges of oral olanzapine 5 to 12.5 mg and fluoxetine 20 to 50 mg. Antidepressant efficacy was demonstrated with olanzapine and fluoxetine in combination in adult patients with a dose range of olanzapine 6 to 12 mg and fluoxetine 25 to 50 mg. Safety of coadministration of doses above 18 mg olanzapine with 75 mg fluoxetine has not been evaluated in clinical studies.
Children and Adolescents (10 to 17 years of age)
****Oral olanzapine should be administered in combination with fluoxetine once daily in the evening, without regard to meals, generally beginning with 2.5 mg of oral olanzapine and 20 mg of fluoxetine. Dosage adjustments, if indicated, can be made according to efficacy and tolerability. Safety of coadministration of doses above 12 mg olanzapine with 50 mg fluoxetine has not been evaluated in pediatric clinical studies.
Safety and efficacy of olanzapine and fluoxetine in combination was determined in clinical trials supporting approval of Symbyax (fixed dose combination of olanzapine and fluoxetine). Symbyax is dosed between 3 mg/25 mg (olanzapine/fluoxetine) per day and 12 mg/ 50 mg (olanzapine/fluoxetine) per day. The following table demonstrates the appropriate individual component doses of olanzapine and fluoxetine versus Symbyax. Dosage adjustments, if indicated, should be made with the individual components according to efficacy and tolerability.
Table 1: Approximate Dose Correspondence Between Symbyaxa and the Combination of Olanzapine and Fluoxetine
a Symbyax (olanzapine/fluoxetine HCl) is a fixed-dose combination of olanzapine and fluoxetine.
** For Symbyax**
** (mg/day)**** Use in Combination**
** Olanzapine**
** (mg/day)**** Fluoxetine**
** (mg/day)**3 mg olanzapine/25 mg fluoxetine
2.5
20
6 mg olanzapine/25 mg fluoxetine
5
20
12 mg olanzapine/25 mg fluoxetine
10+2.5
20
6 mg olanzapine/50 mg fluoxetine
5
40+10
12 mg olanzapine/50 mg fluoxetine
10+2.5
40+10
While there is no body of evidence to answer the question of how long a patient treated with olanzapine and fluoxetine in combination should remain on it, it is generally accepted that bipolar I disorder, including the depressive episodes associated with bipolar I disorder, is a chronic illness requiring chronic treatment. The healthcare provider should periodically reexamine the need for continued pharmacotherapy.
Olanzapine monotherapy is not indicated for the treatment of depressive episodes associated with bipolar I disorder.
2.6 Olanzapine and Fluoxetine in Combination: Treatment Resistant
Depression
When using olanzapine and fluoxetine in combination, also refer to the Clinical Studies section of the package insert for Symbyax.
Oral olanzapine should be administered in combination with fluoxetine once daily in the evening, without regard to meals, generally beginning with 5 mg of oral olanzapine and 20 mg of fluoxetine. Dosage adjustments, if indicated, can be made according to efficacy and tolerability within dose ranges of oral olanzapine 5 to 20 mg and fluoxetine 20 to 50 mg. Antidepressant efficacy was demonstrated with olanzapine and fluoxetine in combination in adult patients with a dose range of olanzapine 6 to 18 mg and fluoxetine 25 to 50 mg.
Safety and efficacy of olanzapine in combination with fluoxetine was determined in clinical trials supporting approval of Symbyax (fixed dose combination of olanzapine and fluoxetine). Symbyax is dosed between 3 mg/25 mg (olanzapine/fluoxetine) per day and 12 mg/50 mg (olanzapine/fluoxetine) per day.Table 1above demonstrates the appropriate individual component doses of olanzapine and fluoxetine versus Symbyax. Dosage adjustments, if indicated, should be made with the individual components according to efficacy and tolerability.
While there is no body of evidence to answer the question of how long a patient treated with olanzapine and fluoxetine in combination should remain on it, it is generally accepted that treatment resistant depression (major depressive disorder in adult patients who do not respond to 2 separate trials of different antidepressants of adequate dose and duration in the current episode) is a chronic illness requiring chronic treatment. The healthcare provider should periodically reexamine the need for continued pharmacotherapy.
Safety of coadministration of doses above 18 mg olanzapine with 75 mg fluoxetine has not been evaluated in clinical studies.
Olanzapine monotherapy is not indicated for treatment of treatment resistant depression (major depressive disorder in patients who do not respond to 2 antidepressants of adequate dose and duration in the current episode).
2.7 Olanzapine and Fluoxetine in Combination: Dosing in Special Populations
The starting dose of oral olanzapine 2.5 to 5 mg with fluoxetine 20 mg should be used for patients with a predisposition to hypotensive reactions, patients with hepatic impairment, or patients who exhibit a combination of factors that may slow the metabolism of olanzapine or fluoxetine in combination (female gender, geriatric age, nonsmoking status), or those patients who may be pharmacodynamically sensitive to olanzapine. Dosing modification may be necessary in patients who exhibit a combination of factors that may slow metabolism. When indicated, dose escalation should be performed with caution in these patients. Olanzapine and fluoxetine in combination have not been systematically studied in patients over 65 years of age or in patients under 10 years of age [seeWarnings and Precautions (5.14),Drug Interactions (7), andClinical Pharmacology (12.3)].
OVERDOSAGE SECTION
10 OVERDOSAGE
10.1 Human Experience
In premarketing trials involving more than 3100 patients and/or normal subjects, accidental or intentional acute overdosage of olanzapine was identified in 67 patients. In the patient taking the largest identified amount, 300 mg, the only symptoms reported were drowsiness and slurred speech. In the limited number of patients who were evaluated in hospitals, including the patient taking 300 mg, there were no observations indicating an adverse change in laboratory analytes or ECG. Vital signs were usually within normal limits following overdoses.
In postmarketing reports of overdose with olanzapine alone, symptoms have been reported in the majority of cases. In symptomatic patients, symptoms with ≥10% incidence included agitation/aggressiveness, dysarthria, tachycardia, various extrapyramidal symptoms, and reduced level of consciousness ranging from sedation to coma. Among less commonly reported symptoms were the following potentially medically serious reactions: aspiration, cardiopulmonary arrest, cardiac arrhythmias (such as supraventricular tachycardia and 1 patient experiencing sinus pause with spontaneous resumption of normal rhythm), delirium, possible neuroleptic malignant syndrome, respiratory depression/arrest, convulsion, hypertension, and hypotension. Eli Lilly and Company has received reports of fatality in association with overdose of olanzapine alone. In 1 case of death, the amount of acutely ingested olanzapine was reported to be possibly as low as 450 mg of oral olanzapine; however, in another case, a patient was reported to survive an acute olanzapine ingestion of approximately 2 g of oral olanzapine.
10.2 Management of Overdose
There is no specific antidote to an overdose of olanzapine. The possibility of multiple drug involvement should be considered. Establish and maintain an airway and ensure adequate oxygenation and ventilation. Cardiovascular monitoring should commence immediately and should include continuous electrocardiographic monitoring to detect possible arrhythmias.
Contact a Certified Poison Control Center for the most up to date information on the management of overdosage (1-800-222-1222).
For specific information about overdosage with lithium or valproate, refer to the Overdosage section of the prescribing information for these products. For specific information about overdosage with olanzapine and fluoxetine in combination, refer to the Overdosage section of the Symbyax prescribing information.
SPL MEDGUIDE SECTION
** MEDICATION GUIDE**
** OLANZAPINE (oh-LAN-za-peen) ORALLY DISINTEGRATING TABLETS, USP**Read the Medication Guide that comes with Olanzapine Orally Disintegrating Tablets before you start taking it and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking to your doctor about your medical condition or treatment. Talk with your doctor or pharmacist if there is something you do not understand or you want to learn more about olanzapine.
** What is the most important information I should know about olanzapine?**
** Olanzapine may cause serious side effects, including:**
** 1.**** Increased risk of death in elderly people who are confused, have memory loss and have lost touch with reality (dementia-related psychosis).**
** 2.**** High blood sugar (hyperglycemia).**
** 3.**** High fat levels in your blood (increased cholesterol and triglycerides), especially in teenagers age 13 to 17 or when used in combination with fluoxetine in children age 10 to 17.**
** 4.**** Weight gain, especially in teenagers age 13 to 17 or when used in combination with fluoxetine in children age 10 to 17.**
** These serious side effects are described below.**
** 1. Increased risk of death in elderly people who are confused, have memory loss and have lost touch with reality (dementia-related psychosis).** Olanzapine is not approved for treating psychosis in elderly people with dementia.
** 2. High blood sugar (hyperglycemia).** High blood sugar can happen if you have diabetes already or if you have never had diabetes. High blood sugar could lead to:
- a build up of acid in your blood due to ketones (ketoacidosis)
- coma
- death
Your doctor should do tests to check your blood sugar before you start taking olanzapine and during treatment. In people who do not have diabetes, sometimes high blood sugar goes away when olanzapine is stopped. People with diabetes and some people who did not have diabetes before taking olanzapine need to take medicine for high blood sugar even after they stop taking olanzapine.
If you have diabetes, follow your doctor's instructions about how often to check your blood sugar while taking olanzapine.
** Call your doctor** if you have any of these symptoms of high blood sugar (hyperglycemia) while taking olanzapine:
- feel very thirsty
- need to urinate more than usual
- feel very hungry
- feel weak or tired
- feel sick to your stomach
- feel confused, or your breath smells fruity
** 3. High fat levels in your blood (cholesterol and triglycerides).** High fat levels may happen in people treated with olanzapine, especially in teenagers (13 to 17 years old), or when used in combination with fluoxetine in children (10 to 17 years old). You may not have any symptoms, so your doctor should do blood tests to check your cholesterol and triglyceride levels before you start taking olanzapine and during treatment.
** 4. Weight gain** . Weight gain is very common in people who take olanzapine. Teenagers (13 to 17 years old) are more likely to gain weight and to gain more weight than adults. Children (10 to 17 years old) are also more likely to gain weight and to gain more weight than adults when olanzapine is used in combination with fluoxetine. Some people may gain a lot of weight while taking olanzapine, so you and your doctor should check your weight regularly. Talk to your doctor about ways to control weight gain, such as eating a healthy, balanced diet, and exercising.
** What is Olanzapine?**
** Olanzapine is a prescription medicine used to treat:**
- schizophrenia in people age 13 or older.
- bipolar disorder, including:
• manic or mixed episodes that happen with bipolar I disorder in people age 13 or older.
• manic or mixed episodes that happen with bipolar I disorder, when used with the medicine lithium or valproate, in adults.
• long-term treatment of bipolar I disorder in adults.
- episodes of depression that happen with bipolar I disorder, when used with the medicine fluoxetine (Prozac®), in people age 10 or older.
- episodes of depression that do not get better after 2 other medicines, also called treatment resistant depression, when used with the medicine fluoxetine (Prozac), in adults.
Olanzapine has not been approved for use in children under 13 years of age. Olanzapine in combination with fluoxetine has not been approved for use in children under 10 years of age.
The symptoms of schizophrenia include hearing voices, seeing things that are not there, having beliefs that are not true, and being suspicious or withdrawn.
The symptoms of bipolar I disorder include alternating periods of depression and high or irritable mood, increased activity and restlessness, racing thoughts, talking fast, impulsive behavior, and a decreased need for sleep.
The symptoms of treatment resistant depression include decreased mood, decreased interest, increased guilty feelings, decreased energy, decreased concentration, changes in appetite, and suicidal thoughts or behavior.
Some of your symptoms may improve with treatment. If you do not think you are getting better, call your doctor.
** What should I tell my doctor before taking olanzapine?**
Olanzapine may not be right for you. Before starting olanzapine, tell your doctor if you have or had:
- heart problems
- seizures
- diabetes or high blood sugar levels (hyperglycemia)
- high cholesterol or triglyceride levels in your blood liver problems
- low or high blood pressure
- strokes or "mini-strokes" also called transient ischemic attacks (TIAs)
- Alzheimer's disease
- narrow-angle glaucoma
- enlarged prostate in men
- bowel obstruction
- phenylketonuria, because olanzapine orally disintegrating tablets contain phenylalanine
- breast cancer
- thoughts of suicide or hurting yourself
- any other medical condition
- are pregnant or plan to become pregnant. It is not known if olanzapine will harm your unborn baby.
• If you become pregnant while receiving olanzapine, talk to your healthcare provider about registering with the National Pregnancy Registry for Atypical Antipsychotics. You can register by calling 1-866-961-2388 or go to http://womensmentalhealth.org/clinical-andresearch- programs/pregnancyregistry/.
- are breast-feeding or plan to breast-feed. Olanzapine passes into your breast milk. Talk to your doctor about the best way to feed your baby if you take olanzapine.
Tell your doctor if you exercise a lot or are in hot places often.
The symptoms of bipolar I disorder, treatment resistant depression or schizophrenia may include** thoughts of suicide** or of hurting yourself or others. If you have these thoughts at any time, tell your doctor or go to an emergency room right away.
** Tell your doctor about all the medicines that you take** , including prescription and nonprescription medicines, vitamins, and herbal supplements. Olanzapine and some medicines may interact with each other and may not work as well, or cause possible serious side effects. Your doctor can tell you if it is safe to take olanzapine with your other medicines. Do not start or stop any medicine while taking olanzapine without talking to your doctor first.
** How should I take Olanzapine Orally Disintegrating Tablets?**
• Take olanzapine exactly as prescribed. Your doctor may need to change (adjust) the dose of olanzapine until it is right for you.
• If you miss a dose of olanzapine, take the missed dose as soon as you remember. If it is almost time for the next dose, just skip the missed dose and take your next dose at the regular time. Do not take two doses of olanzapine at the same time.
•** To prevent serious side effects, do not stop taking olanzapine suddenly. If you need to stop taking olanzapine, your doctor can tell you how to safely stop taking it.**
•** If you take too much olanzapine, call your doctor or poison control center at 1-800-222-1222 right away, or get emergency treatment** .
• Olanzapine can be taken with or without food.
• Olanzapine is usually taken one time each day.
• Take olanzapine orally disintegrating tablets as follows:
- Be sure that your hands are dry.
- Peel back the foil on the blister. Do not push the tablet through the foil.
- As soon as you open the blister, remove the tablet and put it into your mouth.
- The tablet will disintegrate quickly in your saliva so that you can easily swallow it with or without drinking liquid.
• Call your doctor if you do not think you are getting better or have any concerns about your condition while taking olanzapine.
** What should I avoid while taking olanzapine?**
• Olanzapine can cause sleepiness and may affect your ability to make decisions, think clearly, or react quickly. You should not drive, operate heavy machinery, or do other dangerous activities until you know how olanzapine affects you.
• Avoid drinking alcohol while taking olanzapine. Drinking alcohol while you take olanzapine may make you sleepier than if you take olanzapine alone.
** What are the possible side effects of olanzapine?**
** Serious side effects may happen when you take olanzapine, including:**
** • See "What is the most important information I should know about olanzapine?", which describes the increased risk of death in elderly people with dementia-related psychosis and the risks of high blood sugar, high cholesterol and triglyceride levels, and weight gain.**
•** Increased incidence of stroke or "mini-strokes" called transient ischemic attacks (TIAs) in elderly people with dementia-related psychosis** (elderly people who have lost touch with reality due to confusion and memory loss). Olanzapine is not approved for these patients.
•** Neuroleptic Malignant Syndrome (NMS):** NMS is a rare but very serious condition that can happen in people who take antipsychotic medicines, including olanzapine. NMS can cause death and must be treated in a hospital. Call your doctor right away if you become severely ill and have any of these symptoms:
•high fever
•excessive sweating
•rigid muscles
•confusion
•changes in your breathing, heartbeat, and blood pressure.
•** Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS):** DRESS can occur with olanzapine. Features of DRESS may include rash, fever, swollen glands and other internal organ involvement such as liver, kidney, lung and heart. DRESS is sometimes fatal; therefore, tell your doctor immediately if you experience any of these signs.
•** Tardive Dyskinesia** : This condition causes body movements that keep happening and that you can not control. These movements usually affect the face and tongue. Tardive dyskinesia may not go away, even if you stop taking olanzapine. It may also start after you stop taking olanzapine. Tell your doctor if you get any body movements that you can not control.
•** Decreased blood pressure when you change positions, with symptoms of dizziness, fast or slow heartbeat, or fainting.**
** • Difficulty swallowing, that can cause food or liquid to get into your lungs.**
** • Seizures: Tell your doctor if you have a seizure during treatment with olanzapine.**
** • Problems with control of body temperature** : You could become very hot, for instance when you exercise a lot or stay in an area that is very hot. It is important for you to drink water to avoid dehydration. Call your doctor right away if you become severely ill and have any of these symptoms of dehydration:
• sweating too much or not at all
• dry mouth
• feeling very hot
• feeling thirsty
• not able to produce urine.
** Common side effects of olanzapine include:** lack of energy, dry mouth, increased appetite, sleepiness, tremor (shakes), having hard or infrequent stools, dizziness, changes in behavior, or restlessness.
** Other common side effects in teenagers (13 to 17 years old) include** : headache, stomach-area (abdominal) pain, pain in your arms or legs, or tiredness. Teenagers experienced greater increases in prolactin, liver enzymes, and sleepiness, as compared with adults.
Tell your doctor about any side effect that bothers you or that does not go away.
These are not all the possible side effects with olanzapine. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
** How should I store Olanzapine Orally Disintegrating Tablets?**
• Store Olanzapine Orally Disintegrating Tablets at room temperature, between 68°F to 77°F (20°C to 25°C).
• Keep Olanzapine Orally Disintegrating Tablets away from light.
• Keep Olanzapine Orally Disintegrating Tablets dry and away from moisture.
** Keep Olanzapine Orally Disintegrating Tablets and all medicines out of the reach of children.**
** General information about Olanzapine Orally Disintegrating Tablets**
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use olanzapine for a condition for which it was not prescribed. Do not give olanzapine to other people, even if they have the same condition. It may harm them.
This Medication Guide summarizes the most important information about Olanzapine Orally Disintegrating Tablets. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about olanzapine that was written for healthcare professionals.
For more information about Olanzapine Orally Disintegrating Tablets call Strides Pharma Inc. at 1-877-244-9825 or visit www.strides.com
** What are the ingredients in Olanzapine Orally Disintegrating Tablets?**
** Active ingredient** : olanzapine, USP
** Inactive ingredients** : aspartame, crospovidone, magnesium stearate, mannitol, silicone dioxide, sorbitol, talc, and artificial pineapple flavor.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Distributed by:
** Strides Pharma Inc.**
East Brunswick, NJ 08816
SYMBYAX® and PROZAC® are the registered trademarks of Eli Lilly and Company.
** Medication Guide available at: www.strides.com/medication-guides**
R02/2023
PD320-01-1-07
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Olanzapine Orally Disintegrating Tablets, USP 5 mg, 10 mg, 15 mg and 20 mg are available in child resistant unit dose packs of 30 tablets (3 blistercards each containing 10 tablets) as follows:
- Olanzapine orally disintegrating tablets, USP 5 mg are yellow, round, flat faced, beveled edge tablets debossed "P" on one side of the tablet and "320" on the other. (NDC 64380-172-02).
- Olanzapine orally disintegrating tablets, USP 10 mg are yellow, round, flat faced, beveled edge tablets debossed "P" on one side of the tablet and "321" on the other. (NDC 64380-173-02).
- Olanzapine orally disintegrating tablets, USP 15 mg are yellow, round, flat faced, beveled edge tablets debossed "P" on one side of the tablet and "322" on the other. (NDC 64380-174-02).
- Olanzapine orally disintegrating tablets, USP 20 mg are yellow, round, flat faced, beveled edge tablets debossed "P" on one side of the tablet and "323" on the other. (NDC 64380-175-02)
16.2 Storage and Handling
Store olanzapine orally disintegrating tablets at controlled room temperature, 20° to 25°C (68° to 77°F) [see USP].
The USP defines controlled room temperature as a temperature maintained thermostatically that encompasses the usual and customary working environment of 20° to 25°C (68° to 77°F); that results in a mean kinetic temperature calculated to be not more than 25°C; and that allows for excursions between 15° and 30°C (59° and 86°F) that are experienced in pharmacies, hospitals, and warehouses.
Protect olanzapine orally disintegrating tablets from light and moisture.
