Fluticasone Propionate
These highlights do not include all the information needed to use safely and effectively. See full prescribing information for . Initial U.S. Approval: 1990
96e0681d-3788-49cc-8c0e-3185675b8da1
HUMAN PRESCRIPTION DRUG LABEL
Jul 17, 2023
Bryant Ranch Prepack
DUNS: 171714327
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Fluticasone Propionate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Drug Labeling Information
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
•
HPA Axis Suppression and Other Adverse Endocrine Effects [see Warnings and Precautions (5.1)]
•
Local Adverse Reactions [see Warnings and Precautions (5.2)]
•
Concomitant Skin Infections [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In controlled clinical trials, the total incidence of adverse reactions associated with the use of fluticasone propionate ointment was approximately 4%. These adverse reactions were usually mild, self-limiting, and consisted primarily of pruritus, burning, hypertrichosis, increased erythema, urticaria, irritation, and lightheadedness. Each of these events occurred individually in less than 1% of subjects.
6.2 Postmarketing Experience
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following local adverse reactions have been identified during post- approval use of fluticasone propionate ointment: acneiform dermatitis, edema, rash, hypoaesthesia, pustular psoriasis, skin atrophy.
The following systemic adverse reactions have been identified during post- approval use of fluticasone propionate ointment: immunosuppression/Pneumocystis jirovecii/pneumonia/leukopenia/thrombocytopenia; hyperglycemia/glycosuria; Cushing syndrome; generalized body edema/blurred vision; and acute urticarial reaction (edema, urticaria, pruritus, and throat swelling).
The following local adverse reactions have also been reported with the use of topical corticosteroids: telangiectasia, striae, dryness, folliculitis, hypopigmentation, perioral dermatitis, allergic contact dermatitis, secondary infection, and miliaria.
Ophthalmic adverse reactions including cataracts, glaucoma and increased intraocular pressure have been reported with the use of topical corticosteroids.
The most common adverse reactions (<1%) were pruritus, burning, hypertrichosis, increased erythema, urticaria, irritation, and lightheadedness. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Padagis® at 1-866-634-9120 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DESCRIPTION SECTION
11 DESCRIPTION
Fluticasone Propionate Ointment, 0.005% contains fluticasone propionate [S-Fluoromethyl 6α, 9α-difluoro-11β-hydroxy-16α-methyl-3-oxo-17α-propionyloxyandrosta-1,4-diene-17β-carbothioate], a synthetic fluorinated corticosteroid, for topical use.
Chemically, fluticasone propionate is C25H31F3O5S. It has the following structural formula:
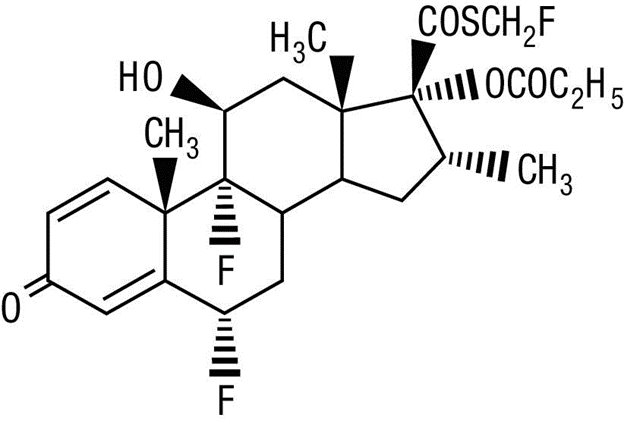
Fluticasone propionate has a molecular weight of 500.6. It is a white to off- white powder and is insoluble in water.
Each gram of fluticasone propionate ointment contains fluticasone propionate 0.05 mg in a white to off-white translucent ointment base of liquid paraffin, microcrystalline wax, propylene glycol, and sorbitan sesquioleate.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Corticosteroids play a role in cellular signaling, immune function, inflammation, and protein regulation; however, the precise mechanism of action of fluticasone propionate ointment in corticosteroid-responsive dermatoses is unknown.
12.2 Pharmacodynamics
Vasoconstrictor Assay
Studies performed with fluticasone propionate ointment indicate that it is in the medium range of potency as demonstrated in vasoconstrictor trials in healthy subjects when compared with other topical corticosteroids. However, similar blanching scores do not necessarily imply therapeutic equivalence.
12.3 Pharmacokinetics
Absorption
The extent of percutaneous absorption of topical corticosteroids is determined by many factors, including the vehicle and the integrity of the epidermal barrier. Occlusive dressing enhances penetration. Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes in the skin increase percutaneous absorption.
In a study of 6 healthy subjects applying 25 g of fluticasone propionate ointment 0.005% twice daily to the trunk and legs for up to 5 days under occlusion, plasma levels of fluticasone ranged from 0.08 to 0.22 ng/mL.
Distribution
The percentage of fluticasone propionate bound to human plasma proteins averaged 91%. Fluticasone propionate is weakly and reversibly bound to erythrocytes. Fluticasone propionate is not significantly bound to human transcortin.
Metabolism
No metabolites of fluticasone propionate were detected in an in vitro study of radiolabeled fluticasone propionate incubated in human skin homogenate.
Fluticasone propionate is metabolized in the liver by cytochrome P450 3A4-mediated hydrolysis of the 5-fluoromethyl carbothioate grouping. This transformation occurs in 1 metabolic step to produce the inactive 17-β-carboxylic acid metabolite, the only known metabolite detected in man. This metabolite has approximately 2,000 times less affinity than the parent drug for the glucocorticoid receptor of human lung cytosol in vitro and negligible pharmacological activity in animal studies. Other metabolites detected in vitro using cultured human hepatoma cells have not been detected in man.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In an oral (gavage) mouse carcinogenicity study, doses of 0.1, 0.3, and 1 mg/kg/day fluticasone propionate were administered to mice for 18 months. Fluticasone propionate demonstrated no tumorigenic potential at oral doses up to 1 mg/kg/day in this study.
In a dermal mouse carcinogenicity study, 0.05% fluticasone propionate ointment (40 μl) was topically administered for 1, 3 or 7 days/week for 80 weeks. Fluticasone propionate demonstrated no tumorigenic potential at dermal doses up to 6.7 μg/kg/day in this study.
Fluticasone propionate was not mutagenic or clastogenic in five in vitro genotoxicity tests (Ames assay, E. coli fluctuation test, S. cerevisiae gene conversion test, Chinese hamster ovary cell chromosome aberration assay and human lymphocyte chromosome aberration assay) and one in vivo genotoxicity test (mouse micronucleus assay).
In a fertility study, male and female rats received subcutaneous doses of fluticasone propionate at doses up to 50 μg/kg/day. No impairment of fertility or mating performance was observed.
