Crest
Crest 3D White Brilliance Luminous Purple
fbfcd837-4d88-5f82-e053-6394a90afbf6
HUMAN OTC DRUG LABEL
Sep 30, 2025
The Procter & Gamble Manufacturing Company
DUNS: 004238200
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Sodium Fluoride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (18)
Drug Labeling Information
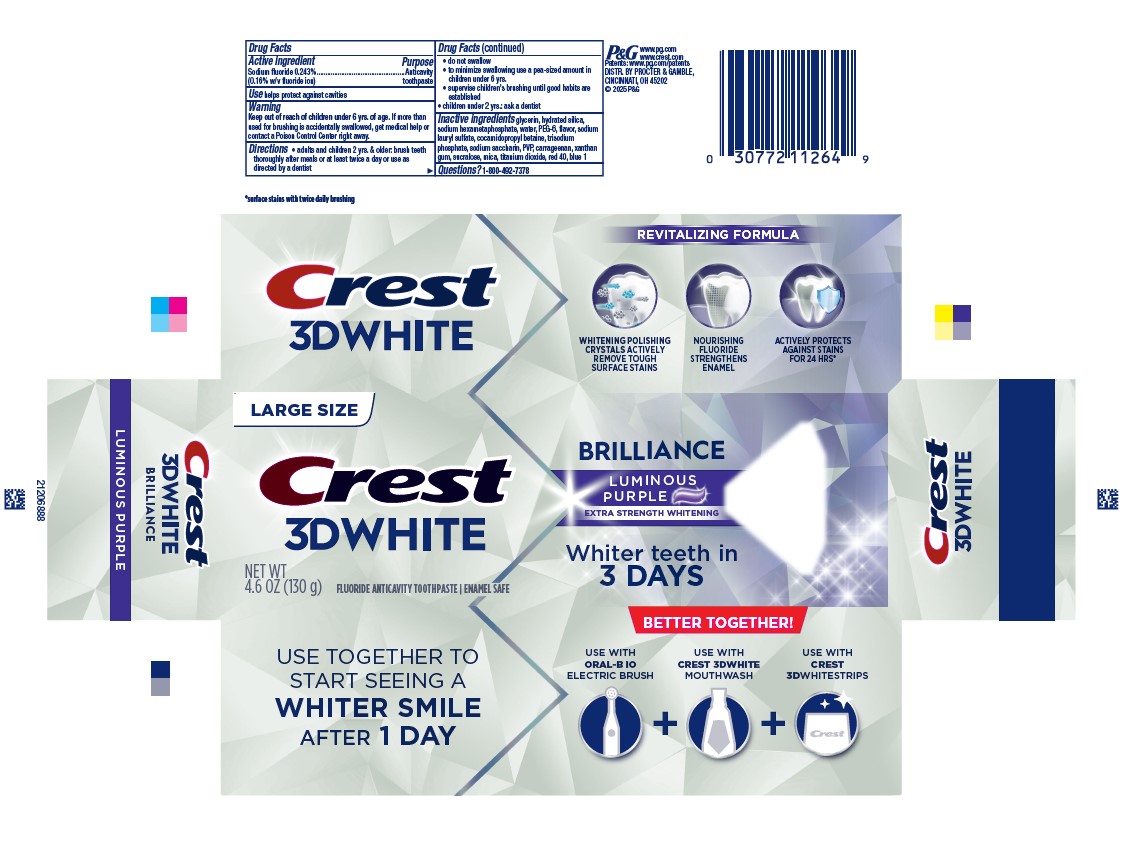
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 130 g Tube Carton
LARGE SIZE
CREST
3D WHITE
BRILLIANCE
LUMINOUS PURPLE
EXTRA STRENGTH WHITENING
net wt 4.6 oz (130 g)
FLUORIDE ANTICAVITY TOOTHPASTE
ENAMEL SAFE
WHITER TEETH IN 3 DAYS
INDICATIONS & USAGE SECTION
Use
helps protect against cavities
OTC - QUESTIONS SECTION
Questions?
1-800-492-7378
SPL UNCLASSIFIED SECTION
DISTR. BY PROCTER & GAMBLE, CINCINNATI, OH 45202
OTC - ACTIVE INGREDIENT SECTION
Active ingredient
Sodium fluoride 0.243% (0.15% w/v fluoride ion)
OTC - PURPOSE SECTION
Purpose
Anticavity toothpaste
WARNINGS SECTION
Warning
Keep out of reach of children under 6 yrs. of age. If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
Directions
- adults and children 2 yrs. & older: brush teeth thoroughly after meals or at least twice a day or use as directed by a dentist
- do not swallow
- to minimize swallowing use a pea-sized amount in children under 6
- supervise children's brushing until good habits are established
- children under 2 yrs.: ask a dentist
INACTIVE INGREDIENT SECTION
Inactive ingredients
glycerin, hydrated silica, sodium hexametaphosphate, water, PEG-6, flavor,
sodium
lauryl sulfate, cocamidopropyl betaine, trisodium phosphate, sodium saccharin,
PVP, carrageenan, xanthan gum, sucralose, mica, titanium dioxide, red 40, blue
1
