Loteprednol Etabonate
These highlights do not include all the information needed to use LOTEPREDNOL ETABONATE OPHTHALMIC GEL safely and effectively. See full prescribing information for LOTEPREDNOL ETABONATE OPHTHALMIC GEL.LOTEPREDNOL ETABONATE OPHTHALMIC GEL 0.5%, for topical ophthalmic use.Initial U.S. Approval: 1998
7bbdbd31-5a8f-4d69-aa8e-5b700895e804
HUMAN PRESCRIPTION DRUG LABEL
Mar 31, 2021
Bausch & Lomb Incorporated
DUNS: 196603781
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
loteprednol etabonate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
Loteprednol Etabonate Ophthalmic Gel is a corticosteroid indicated for the treatment of post-operative inflammation and pain following ocular surgery.
Loteprednol Etabonate Ophthalmic Gel is a corticosteroid indicated for the treatment of postoperative inflammation and pain following ocular surgery. (1)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
Loteprednol Etabonate Ophthalmic Gel is contraindicated in most viral diseases of the cornea and conjunctiva including epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, and varicella, in mycobacterial infection of the eye and fungal diseases of ocular structures.
Loteprednol Etabonate Ophthalmic Gel is contraindicated in most viral diseases of the cornea and conjunctiva including epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, and varicella, in mycobacterial infection of the eye and fungal diseases of ocular structures. (4)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
Adverse reactions associated with ophthalmic steroids include elevated intraocular pressure, which may be associated with infrequent optic nerve damage, visual acuity and field defects, posterior subcapsular cataract formation, delayed wound healing and secondary ocular infection from pathogens including herpes simplex, and perforation of the globe where there is thinning of the cornea or sclera.
The most common adverse drug reactions reported in the clinical trials (2-5%) were anterior chamber inflammation, eye pain, and foreign body sensation.
The most common adverse drug reactions (2-5%) were anterior chamber inflammation, eye pain, and foreign body sensation. (6)
**To report SUSPECTED ADVERSE REACTIONS, contactBausch & Lomb Incorporated at 1‑800-321-4576 or FDA at 1-800-FDA-1088 or **www.fda.gov/medwatch.
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
Loteprednol Etabonate Ophthalmic Gel is a sterile preserved ophthalmic gel containing 5 mg of loteprednol etabonate per gram of gel.
Loteprednol Etabonate Ophthalmic Gel is a sterile preserved ophthalmic gel containing 5 mg of loteprednol etabonate per gram of gel. (3)
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
Invert closed bottle and shake once to fill tip before instilling drops.
Apply one to two drops of Loteprednol Etabonate Ophthalmic Gel into the conjunctival sac of the affected eye four times daily beginning the day after surgery and continuing throughout the first 2 weeks of the post-operative period.
•
Invert closed bottle and shake once to fill tip before instilling drops. (2)
•
Apply one to two drops of Loteprednol Etabonate Ophthalmic Gel into the conjunctival sac of the affected eye four times daily beginning the day after surgery and continuing throughout the first 2 weeks of the postoperative period. (2)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies with loteprednol etabonate in pregnant women.
Loteprednol etabonate produced teratogenicity at clinically relevant doses in the rabbit and rat when administered orally during pregnancy. Loteprednol etabonate produced malformations when administered orally to pregnant rabbits at doses ≥ 1.2 times the recommended human ophthalmic dose (RHOD) and to pregnant rats at doses ≥ 30 times the RHOD. In pregnant rats receiving oral doses of loteprednol etabonate during the period equivalent to the last trimester of pregnancy through lactation in humans, survival of offspring was reduced at doses ≥ 3 times the RHOD. Maternal toxicity was observed in rats at doses ≥ 304 times the RHOD, and a maternal no observed adverse effect level (NOAEL) was established at 30 times the RHOD.
The background risk of major birth defects and miscarriage for the indicated population is unknown. However, the background risk in the U.S. general population of major birth defects is 2 to 4%, and of miscarriage is 15 to 20%, of clinically recognized pregnancies.
Data
Animal Data
Embryofetal studies were conducted in pregnant rabbits administered loteprednol etabonate by oral gavage on gestation days 6 to 18, to target the period of organogenesis. Loteprednol etabonate produced fetal malformations at doses ≥ 0.1 mg/kg (1.2 times the recommended human ophthalmic dose (RHOD) based on body surface area, assuming 100% absorption). Spina bifida (including meningocele) was observed at doses ≥ 0.1 mg/kg, and exencephaly and craniofacial malformations were observed at doses ≥ 0.4 mg/kg (4.9 times the RHOD). At 3 mg/kg (36 times the RHOD), loteprednol etabonate was associated with increased incidences of abnormal left common carotid artery, limb flexures, umbilical hernia, scoliosis, and delayed ossification. Abortion and embryofetal lethality (resorption) occurred at doses ≥ 6 mg/kg (73 times the RHOD). A NOAEL for developmental toxicity was not established in this study. The NOAEL for maternal toxicity in rabbits was 3 mg/kg/day.
Embryofetal studies were conducted in pregnant rats administered loteprednol etabonate by oral gavage on gestation days 6 to 15, to target the period of organogenesis. Loteprednol etabonate produced fetal malformations, including absent innominate artery at doses ≥ 5 mg/kg (30 times the RHOD); and cleft palate, agnathia, cardiovascular defects, umbilical hernia, decreased fetal body weight and decreased skeletal ossification at doses ≥ 50 mg/kg (304 times the RHOD). Embryofetal lethality (resorption) was observed at 100 mg/kg (608 times the RHOD). The NOAEL for developmental toxicity in rats was 0.5 mg/kg (3 times the RHOD). Loteprednol etabonate was maternally toxic (reduced body weight gain) at doses of ≥ 50 mg/kg/day. The NOAEL for maternal toxicity was 5 mg/kg.
A peri-/postnatal study was conducted in rats administered loteprednol etabonate by oral gavage from gestation day 15 (start of fetal period) to postnatal day 21 (the end of lactation period). At doses ≥ 0.5 mg/kg (3 times the clinical dose), reduced survival was observed in live-born offspring. Doses ≥ 5 mg/kg (30 times the RHOD) caused umbilical hernia/incomplete gastrointestinal tract. Doses ≥ 50 mg/kg (304 times the RHOD) produced maternal toxicity (reduced body weight gain, death), decreased number of live- born offspring, decreased birth weight, and delays in postnatal development. A developmental NOAEL was not established in this study. The NOAEL for maternal toxicity was 5 mg/kg.
8.2 Lactation
There are no data on the presence of loteprednol etabonate in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered, along with the mother’s clinical need for LOTEMAX and any potential adverse effects on the breastfed infant from LOTEMAX.
8.4 Pediatric Use
The safety and effectiveness of LOTEMAX have been established in the pediatric population. Use of LOTEMAX in this population is supported by evidence from adequate and well-controlled trials of LOTEMAX in adults with additional data from a safety and efficacy trial in pediatric patients from birth to 11 years of age [see Clinical Studies (14)].
8.5 Geriatric Use
No overall differences in safety and effectiveness have been observed between elderly and younger patients.
DESCRIPTION SECTION
11 DESCRIPTION
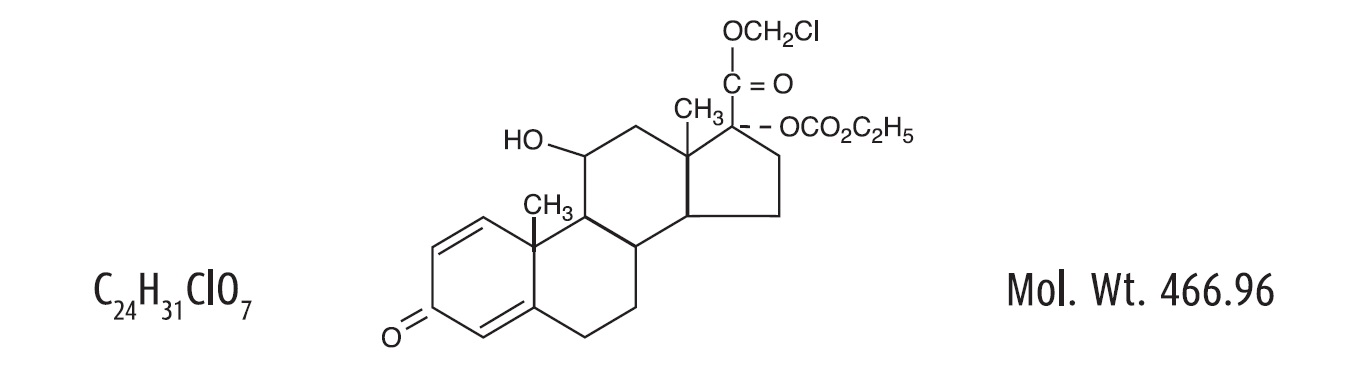
Loteprednol etabonate is a corticosteroid. Its chemical name is chloromethyl 17α-[(ethoxycarbonyl)oxy]-11β-hydroxy-3-oxoandrosta-1,4-diene-17β-carboxylate. Its molecular formula is C24H31ClO7 and its chemical structure is:
Loteprednol Etabonate Ophthalmic Gel 0.5% contains a sterile, topical corticosteroid for ophthalmic use. Loteprednol etabonate is a white to off- white powder.
Each gram contains:
•
ACTIVE: loteprednol etabonate 5 mg (0.5%)
•
INACTIVES: boric acid, edetate disodium dihydrate, glycerin, polycarbophil, propylene glycol, sodium chloride, tyloxapol, water for injection, and sodium hydroxide to adjust to a pH of between 6 and 7
•
PRESERVATIVE: benzalkonium chloride 0.003%
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Corticosteroids inhibit the inflammatory response to a variety of inciting agents and probably delay or slow healing. They inhibit the edema, fibrin deposition, capillary dilation, leukocyte migration, capillary proliferation, fibroblast proliferation, deposition of collagen, and scar formation associated with inflammation. While glucocorticoids are known to bind to and activate the glucocorticoid receptor, the molecular mechanisms involved in glucocorticoid/glucocorticoid receptor‑dependent modulation of inflammation are not clearly established. However, corticosteroids are thought to inhibit prostaglandin production through several independent mechanisms.
12.3 Pharmacokinetics
Loteprednol etabonate is lipid soluble and can penetrate into cells. Loteprednol etabonate is synthesized through structural modifications of prednisolone-related compounds so that it will undergo a predictable transformation to an inactive metabolite. Based upon in vivoand in vitropreclinical metabolism studies, loteprednol etabonate undergoes extensive metabolism to the inactive carboxylic acid metabolites, PJ-91 and PJ-90. The systemic exposure to loteprednol etabonate following ocular administration of Loteprednol Etabonate Ophthalmic Gel has not been studied in humans.
