EXEMESTANE
These highlights do not include all the information needed to use EXEMESTANE safely and effectively. See full prescribing information for EXEMESTANE. EXEMESTANE tablets, for oral use Initial U.S. Approval: 1999
69abd0ff-b200-4d1a-b14a-80e980a8e781
HUMAN PRESCRIPTION DRUG LABEL
Sep 8, 2023
Greenstone LLC
DUNS: 825560733
Mylan Pharmaceuticals Inc.
DUNS: 059295980
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Exemestane
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (15)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 25 mg Tablet Bottle Label
NDC 59762-2858-1
30 Tablets
GREENSTONE**®**** BRAND**
exemestane
tablets
25 mg
Rx only

WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Reductions in Bone Mineral Density (BMD)
Reductions in bone mineral density (BMD) over time are seen with exemestane use. Table 1 describes changes in BMD from baseline to 24 months in patients receiving exemestane compared to patients receiving tamoxifen (IES) or placebo (027). Concomitant use of bisphosphonates, vitamin D supplementation, and calcium was not allowed.
Table 1. Percent Change in BMD from Baseline to 24 months, Exemestane vs. Control1|
IES |
027 | |||
|
BMD |
Exemestane |
Tamoxifen****1 |
Exemestane |
Placebo****1 |
|
Lumbar spine (%) |
-3.1 |
-0.2 |
-3.5 |
-2.4 |
|
Femoral neck (%) |
-4.2 |
-0.3 |
-4.6 |
-2.6 |
During adjuvant treatment with exemestane, women with osteoporosis or at risk of osteoporosis should have their bone mineral density formally assessed by bone densitometry at the commencement of treatment. Monitor patients for bone mineral density loss and treat as appropriate.
5.2 Vitamin D Assessment
Routine assessment of 25-hydroxy vitamin D levels prior to the start of aromatase inhibitor treatment should be performed, due to the high prevalence of vitamin D deficiency in women with early breast cancer (EBC). Women with vitamin D deficiency should receive supplementation with vitamin D.
5.3 Administration with Estrogen-Containing Agents
EXEMESTANE should not be coadministered with systemic estrogen-containing agents as these could interfere with its pharmacologic action.
5.4 Laboratory Abnormalities
In patients with early breast cancer, the incidence of hematological abnormalities of Common Toxicity Criteria (CTC) grade ≥1 was lower in the exemestane treatment group, compared with tamoxifen. Incidence of CTC grade 3 or 4 abnormalities was low (approximately 0.1%) in both treatment groups. Approximately 20% of patients receiving exemestane in clinical studies in advanced breast cancer experienced CTC grade 3 or 4 lymphocytopenia. Of these patients, 89% had a pre-existing lower grade lymphopenia. Forty percent of patients either recovered or improved to a lesser severity while on treatment. Patients did not have a significant increase in viral infections, and no opportunistic infections were observed. Elevations of serum levels of AST, ALT, alkaline phosphatase, and gamma glutamyl transferase >5 times the upper value of the normal range (i.e., ≥ CTC grade 3) have been rarely reported in patients treated for advanced breast cancer but appear mostly attributable to the underlying presence of liver and/or bone metastases. In the comparative study in advanced breast cancer patients, CTC grade 3 or 4 elevation of gamma glutamyl transferase without documented evidence of liver metastasis was reported in 2.7% of patients treated with EXEMESTANE and in 1.8% of patients treated with megestrol acetate.
In patients with early breast cancer, elevations in bilirubin, alkaline phosphatase, and creatinine were more common in those receiving exemestane than either tamoxifen or placebo. Treatment-emergent bilirubin elevations (any CTC grade) occurred in 5% of exemestane patients and 0.8% of tamoxifen patients on the Intergroup Exemestane Study (IES), and in 7% of exemestane treated patients vs. 0% of placebo treated patients in the 027 study. CTC grade 3–4 increases in bilirubin occurred in 0.9% of exemestane treated patients compared to 0.1% of tamoxifen treated patients. Alkaline phosphatase elevations of any CTC grade occurred in 15% of exemestane treated patients on the IES compared to 2.6% of tamoxifen treated patients, and in 14% of exemestane treated patients compared to 7% of placebo treated patients in study 027. Creatinine elevations occurred in 6% of exemestane treated patients and 4.3% of tamoxifen treated patients on the IES and in 6% of exemestane treated patients and 0% of placebo treated patients in study 027.
5.5 Use in Premenopausal Women
EXEMESTANE is not indicated for the treatment of breast cancer in premenopausal women.
5.6 Embryo-Fetal Toxicity
Based on findings from animal studies and its mechanism of action, EXEMESTANE can cause fetal harm when administered to a pregnant woman. In animal reproduction studies, administration of exemestane to pregnant rats and rabbits caused increased incidence of abortions and embryo-fetal toxicity. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with EXEMESTANE and for 1 month after the last dose [see Use in Specific Populations (8.1), (8.3) and Clinical Pharmacology (12.1)].
•
Reductions in bone mineral density (BMD) over time are seen with exemestane use (5.1).
•
Routine assessment of 25-hydroxy vitamin D levels prior to the start of aromatase inhibitor treatment should be performed (5.2).
•
Embryo-Fetal Toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and to use effective contraception (5.6, 8.1, 8.3).
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
•
Reductions in Bone Mineral Density (BMD) [see Warnings and Precautions (5.1)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Adjuvant Therapy
The data described below reflect exposure to EXEMESTANE in 2325 postmenopausal women with early breast cancer. EXEMESTANE tolerability in postmenopausal women with early breast cancer was evaluated in two well-controlled trials: the IES study [see Clinical Studies (14.1)] and the 027 study (a randomized, placebo-controlled, double-blind, parallel group study specifically designed to assess the effects of exemestane on bone metabolism, hormones, lipids, and coagulation factors over 2 years of treatment).
The median duration of adjuvant treatment was 27.4 months and 27.3 months for patients receiving EXEMESTANE or tamoxifen, respectively, within the IES study and 23.9 months for patients receiving EXEMESTANE or placebo within the 027 study. Median duration of observation after randomization for EXEMESTANE was 34.5 months and for tamoxifen was 34.6 months. Median duration of observation was 30 months for both groups in the 027 study.
Certain adverse reactions, which were expected based on the known pharmacological properties and side effect profiles of test drugs, were actively sought through a positive checklist. Signs and symptoms were graded for severity using CTC in both studies. Within the IES study, the presence of some illnesses/conditions was monitored through a positive checklist without assessment of severity. These included myocardial infarction, other cardiovascular disorders, gynecological disorders, osteoporosis, osteoporotic fractures, other primary cancer, and hospitalizations.
Within the IES study, discontinuations due to adverse reactions occurred in 6% and 5% of patients receiving EXEMESTANE and tamoxifen, respectively, and in 12% and 4.1% of patients receiving EXEMESTANE or placebo respectively within study 027.
Deaths due to any cause were reported for 1.3% of the exemestane treated patients and 1.4% of the tamoxifen treated patients within the IES study. There were 6 deaths due to stroke on the exemestane arm compared to 2 on tamoxifen. There were 5 deaths due to cardiac failure on the exemestane arm compared to 2 on tamoxifen.
The incidence of cardiac ischemic events (myocardial infarction, angina, and myocardial ischemia) was 1.6% in exemestane treated patients and 0.6% in tamoxifen treated patients in the IES study. Cardiac failure was observed in 0.4% of exemestane treated patients and 0.3% of tamoxifen treated patients.
In the adjuvant treatment of early breast cancer, the most common adverse reactions occurring in ≥10% of patients in any treatment group (EXEMESTANE vs. tamoxifen) were hot flushes (21% vs. 20%), fatigue (16% vs. 15%), arthralgia (15% vs. 9%), headache (13% vs. 11%), insomnia (12% vs. 9%), and increased sweating (12% vs. 10%). Discontinuation rates due to AEs were similar between EXEMESTANE and tamoxifen (6% vs. 5%). Incidences of cardiac ischemic events (myocardial infarction, angina, and myocardial ischemia) were EXEMESTANE 1.6%, tamoxifen 0.6%. Incidence of cardiac failure: EXEMESTANE 0.4%, tamoxifen 0.3%.
Treatment-emergent adverse reactions and illnesses including all causalities and occurring with an incidence of ≥5% in either treatment group of the IES study during or within one month of the end of treatment are shown in Table 2.
Table 2. Incidence (%) of Adverse Reactions of all Grades* and Illnesses Occurring in (≥5%) of Patients in Any Treatment Group in Study IES in Postmenopausal Women with Early Breast Cancer|
% of patients | ||
|---|---|---|
|
Body system and Adverse Reaction by MedDRA dictionary |
EXEMESTANE |
Tamoxifen |
| ||
|
Eye | ||
|
Visual disturbances‡ |
5 |
3.8 |
|
Gastrointestinal | ||
|
Nausea‡ |
9 |
9 |
|
General Disorders | ||
|
Fatigue‡ |
16 |
15 |
|
Musculoskeletal | ||
|
Arthralgia |
15 |
9 |
|
Pain in limb |
9 |
6 |
|
Back pain |
9 |
7 |
|
Osteoarthritis |
6 |
4.5 |
|
Nervous System | ||
|
Headache‡ |
13 |
11 |
|
Dizziness‡ |
10 |
8 |
|
Psychiatric | ||
|
Insomnia‡ |
12 |
9 |
|
Depression |
6 |
6 |
|
Skin & Subcutaneous Tissue | ||
|
Increased sweating‡ |
12 |
10 |
|
Vascular | ||
|
Hot flushes‡ |
21 |
20 |
|
Hypertension |
10 |
8 |
In the IES study, as compared to tamoxifen, EXEMESTANE was associated with a higher incidence of events in musculoskeletal disorders and in nervous system disorders, including the following events occurring with frequency lower than 5% (osteoporosis [4.6% vs. 2.8%], osteochondrosis [0.3% vs. 0%] and stenosing tenosynovitis (trigger finger) [0.3% vs. 0%], paresthesia [2.6% vs. 0.9%], carpal tunnel syndrome [2.4% vs. 0.2%], and neuropathy [0.6% vs. 0.1%]). Diarrhea was also more frequent in the exemestane group (4.2% vs. 2.2%). Clinical fractures were reported in 94 patients receiving exemestane (4.2%) and 71 patients receiving tamoxifen (3.1%). After a median duration of therapy of about 30 months and a median follow-up of about 52 months, gastric ulcer was observed at a slightly higher frequency in the EXEMESTANE group compared to tamoxifen (0.7% vs. <0.1%). The majority of patients on EXEMESTANE with gastric ulcer received concomitant treatment with non-steroidal anti- inflammatory agents and/or had a prior history.
Tamoxifen was associated with a higher incidence of muscle cramps [3.1% vs. 1.5%], thromboembolism [2.0% vs. 0.9%], endometrial hyperplasia [1.7% vs. 0.6%], and uterine polyps [2.4% vs. 0.4%].
Common adverse reactions occurring in study 027 are described in Table 3.
Table 3. Incidence of Selected Treatment-Emergent Adverse Reactions of all CTC Grades* Occurring in ≥5% of Patients in Either Arm in Study 027
| ||
|
Adverse Reaction |
Exemestane |
Placebo |
|
Hot flushes |
33 |
25 |
|
Arthralgia |
29 |
29 |
|
Increased sweating |
18 |
21 |
|
Alopecia |
15 |
4.1 |
|
Hypertension |
15 |
7 |
|
Insomnia |
14 |
15 |
|
Nausea |
12 |
16 |
|
Fatigue |
11 |
19 |
|
Abdominal pain |
11 |
14 |
|
Depression |
10 |
7 |
|
Diarrhea |
10 |
1.4 |
|
Dizziness |
10 |
10 |
|
Dermatitis |
8 |
1.4 |
|
Headache |
7 |
4.1 |
|
Myalgia |
6 |
4.1 |
|
Edema |
6 |
7 |
Treatment of Advanced Breast Cancer
A total of 1058 patients were treated with exemestane 25 mg once daily in the clinical trials program. One death was considered possibly related to treatment with exemestane; an 80-year-old woman with known coronary artery disease had a myocardial infarction with multiple organ failure after 9 weeks on study treatment. In the clinical trials program, 3% of the patients discontinued treatment with exemestane because of adverse reactions, 2.7% of patients discontinued exemestane within the first 10 weeks of treatment.
In the comparative study, adverse reactions were assessed for 358 patients treated with EXEMESTANE and 400 patients treated with megestrol acetate. Fewer patients receiving EXEMESTANE discontinued treatment because of adverse reactions than those treated with megestrol acetate (2% vs. 5%). Adverse reactions that were considered drug related or of indeterminate cause included hot flashes (13% vs. 5%), nausea (9% vs. 5%), fatigue (8% vs. 10%), increased sweating (4% vs. 8%), and increased appetite (3% vs. 6%) for EXEMESTANE and megestrol acetate, respectively. The proportion of patients experiencing an excessive weight gain (>10% of their baseline weight) was significantly higher with megestrol acetate than with EXEMESTANE (17% vs. 8%). In the treatment of advanced breast cancer, the most common adverse reactions included hot flushes (13% vs. 5%), nausea (9% vs. 5%), fatigue (8% vs. 10%), increased sweating (4% vs. 8%), and increased appetite (3% vs. 6%) for EXEMESTANE and megestrol acetate, respectively.
Table 4 shows the adverse reactions of all CTC grades, regardless of causality, reported in 5% or greater of patients in the study treated either with EXEMESTANE or megestrol acetate.
Table 4. Incidence (%) of Adverse Reactions of all Grades* and Causes Occurring in ≥5% of Advanced Breast Cancer Patients In Each Treatment Arm in the Comparative Study|
Body system and Adverse Reaction by WHO ART dictionary |
EXEMESTANE |
Megestrol Acetate |
|---|---|---|
| ||
|
Autonomic Nervous | ||
|
Increased sweating |
6 |
9 |
|
Body as a Whole | ||
|
Fatigue |
22 |
29 |
|
Hot flashes |
13 |
6 |
|
Pain |
13 |
13 |
|
Influenza-like symptoms |
6 |
5 |
|
Edema (includes edema, peripheral edema, leg edema) |
7 |
6 |
|
Cardiovascular | ||
|
Hypertension |
5 |
6 |
|
Nervous | ||
|
Depression |
13 |
9 |
|
Insomnia |
11 |
9 |
|
Anxiety |
10 |
11 |
|
Dizziness |
8 |
6 |
|
Headache |
8 |
7 |
|
Gastrointestinal | ||
|
Nausea |
18 |
12 |
|
Vomiting |
7 |
4 |
|
Abdominal pain |
6 |
11 |
|
Anorexia |
6 |
5 |
|
Constipation |
5 |
8 |
|
Diarrhea |
4 |
5 |
|
Increased appetite |
3 |
6 |
|
Respiratory | ||
|
Dyspnea |
10 |
15 |
|
Coughing |
6 |
7 |
Adverse reactions of any cause (from 2% to 5%) reported in the comparative study for patients receiving EXEMESTANE 25 mg once daily were fever, generalized weakness, paresthesia, pathological fracture, bronchitis, sinusitis, rash, itching, urinary tract infection, and lymphedema.
Additional adverse reactions of any cause observed in the overall clinical trials program (N = 1058) in 5% or greater of patients treated with exemestane 25 mg once daily but not in the comparative study included pain at tumor sites (8%), asthenia (6%), and fever (5%). Adverse reactions of any cause reported in 2% to 5% of all patients treated with exemestane 25 mg in the overall clinical trials program but not in the comparative study included chest pain, hypoesthesia, confusion, dyspepsia, arthralgia, back pain, skeletal pain, infection, upper respiratory tract infection, pharyngitis, rhinitis, and alopecia.
6.2 Post-Marketing Experience
The following adverse reactions have been identified during post approval use of EXEMESTANE. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune system disorders- hypersensitivity
Hepatobiliary disorders- hepatitis including cholestatic hepatitis
Nervous system disorders- paresthesia
Musculoskeletal and connective tissue disorder- tendon disorders including tendon rupture, tendonitis, and tenosynovitis
Skin and subcutaneous tissue disorders- acute generalized exanthematous pustulosis, urticaria, pruritus
•
Early breast cancer: Adverse reactions occurring in ≥10% of patients in any treatment group (EXEMESTANE vs. tamoxifen) were hot flushes (21% vs. 20%), fatigue (16% vs. 15%), arthralgia (15% vs. 9%), headache (13% vs. 11%), insomnia (12% vs. 9%), and increased sweating (12% vs. 10%). Discontinuation rates due to AEs were similar between EXEMESTANE and tamoxifen (6% vs. 5%). Incidences of cardiac ischemic events (myocardial infarction, angina, and myocardial ischemia) were EXEMESTANE 1.6%, tamoxifen 0.6%. Incidence of cardiac failure: EXEMESTANE 0.4%, tamoxifen 0.3% (6, 6.1).
•
Advanced breast cancer: Most common adverse reactions were mild to moderate and included hot flushes (13% vs. 5%), nausea (9% vs. 5%), fatigue (8% vs. 10%), increased sweating (4% vs. 8%), and increased appetite (3% vs. 6%) for EXEMESTANE and megestrol acetate, respectively (6, 6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Greenstone LLC at 1-877-446-3679 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings in animal studies and its mechanism of action, EXEMESTANE can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. Limited human data from case reports are insufficient to inform a drug-associated risk. In animal reproduction studies, administration of exemestane to pregnant rats and rabbits caused increased incidence of abortions, embryo-fetal toxicity, and prolonged gestation with abnormal or difficult labor [see Data]. Advise pregnant women of the potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2–4% and 15–20%, respectively.
Data
Animal Data
In animal reproduction studies in rats and rabbits, exemestane caused embryo- fetal toxicity, and was abortifacient. Radioactivity related to 14C-exemestane crossed the placenta of rats following oral administration of 1 mg/kg exemestane. The concentration of exemestane and its metabolites was approximately equivalent in maternal and fetal blood. When rats were administered exemestane from 14 days prior to mating until either days 15 or 20 of gestation, and resuming for the 21 days of lactation, an increase in placental weight was seen at 4 mg/kg/day (approximately 1.5 times the recommended human daily dose on a mg/m2 basis). Increased resorptions, reduced number of live fetuses, decreased fetal weight, retarded ossification, prolonged gestation and abnormal or difficult labor was observed at doses equal to or greater than 20 mg/kg/day (approximately 7.5 times the recommended human daily dose on a mg/m2 basis). Daily doses of exemestane, given to rabbits during organogenesis, caused a decrease in placental weight at 90 mg/kg/day (approximately 70 times the recommended human daily dose on a mg/m2 basis) and in the presence of maternal toxicity, abortions, an increase in resorptions, and a reduction in fetal body weight were seen at 270 mg/kg/day. No malformations were noted when exemestane was administered to pregnant rats or rabbits during the organogenesis period at doses up to 810 and 270 mg/kg/day, respectively (approximately 320 and 210 times the recommended human dose on a mg/m2 basis, respectively).
8.2 Lactation
Risk Summary
There is no information on the presence of exemestane in human milk, or on its effects on the breastfed infant or milk production. Exemestane is present in rat milk at concentrations similar to maternal plasma (see Data). Because of the potential for serious adverse reactions in breast-fed infants from EXEMESTANE, advise a woman not to breastfeed during treatment with EXEMESTANE and for 1 month after the final dose.
Data
Radioactivity related to exemestane appeared in rat milk within 15 minutes of oral administration of radiolabeled exemestane. Concentrations of exemestane and its metabolites were approximately equivalent in the milk and plasma of rats for 24 hours after a single oral dose of 1 mg/kg 14C-exemestane.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Pregnancy testing is recommended for females of reproductive potential within seven days prior to initiating EXEMESTANE.
Contraception
Females
EXEMESTANE can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment with EXEMESTANE and for 1 month after the final dose.
Infertility
Based on findings in animals, male and female fertility may be impaired by treatment with EXEMESTANE [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
8.6 Hepatic Impairment
The AUC of exemestane was increased in subjects with moderate or severe hepatic impairment (Childs-Pugh B or C) [see Clinical Pharmacology (12.3)]. However, based on experience with exemestane at repeated doses up to 200 mg daily that demonstrated a moderate increase in non life-threatening adverse reactions, dosage adjustment does not appear to be necessary.
8.7 Renal Impairment
The AUC of exemestane was increased in subjects with moderate or severe renal impairment (creatinine clearance <35 mL/min/1.73 m2) [see Clinical Pharmacology (12.3)]. However, based on experience with exemestane at repeated doses up to 200 mg daily that demonstrated a moderate increase in non life- threatening adverse reactions, dosage adjustment does not appear to be necessary.
Lactation: Advise not to breastfeed (8.2).
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
EXEMESTANE tablets are round, biconvex, off-white to slightly greyish sugar- coated tablets, about 6 mm diameter, printed with numbers 2858 on one side in black ink. Each tablet contains 25 mg of exemestane. EXEMESTANE is packaged in HDPE bottles with a child-resistant screw cap, supplied in packs of 30 tablets.
30-tablet HDPE bottle NDC 59762-2858-1
Store at 25°C (77ºF); excursions permitted to 15°–30°C (59°–86°F) [see USP Controlled Room Temperature].
SPL UNCLASSIFIED SECTION

LAB-0444-11.0
SPL PATIENT PACKAGE INSERT SECTION
|
Patient Information | |||
|---|---|---|---|
|
This Patient Information has been approved by the U.S. Food and Drug Administration Revised: 12/2024 | |||
|
What is EXEMESTANE? EXEMESTANE is used in women who are past menopause for the treatment of: | |||
|
• o o o o • | |||
|
It is not known if EXEMESTANE is safe and effective in children. | |||
|
Do not take EXEMESTANE if youare allergic to EXEMESTANE or any of the ingredients in EXEMESTANE. See the end of this leaflet for a complete list of ingredients in EXEMESTANE. | |||
|
Before you take EXEMESTANE, tell your doctor about all your medical conditions, including if you: | |||
|
• • • o o • • | |||
|
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements. Especially tell your doctor if you take medicines that contain estrogen, including other hormone replacement therapy or birth control pills or patches. EXEMESTANE should not be taken with medicines that contain estrogen as they could affect how well EXEMESTANE works. | |||
|
How should I take EXEMESTANE? • • • | |||
|
What are the possible side effects of EXEMESTANE? EXEMESTANE may cause serious side effects, including: | |||
|
• | |||
|
The most common side effects of EXEMESTANE in women with early breast cancer include: | |||
|
• • |
• • |
• • | |
|
The most common side effects of EXEMESTANE in women with advanced breast cancer include: | |||
|
• • |
• • |
• | |
|
Your doctor will do blood tests to check your vitamin D level before starting treatment with EXEMESTANE. EXEMESTANE may cause decreased fertility in males and females. Talk to your doctor if you have concerns about fertility. These are not all the possible side effects of EXEMESTANE. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | |||
|
How should I store EXEMESTANE? • • | |||
|
General information about the safe and effective use of EXEMESTANE. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use EXEMESTANE for a condition for which it was not prescribed. Do not give EXEMESTANE to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or doctor for information about EXEMESTANE that is written for health professionals. | |||
|
What is in EXEMESTANE? Active ingredient: exemestane Inactive ingredients: mannitol, crospovidone, polysorbate 80, hypromellose, colloidal silicon dioxide, microcrystalline cellulose, sodium starch glycolate, magnesium stearate, simethicone, polyethylene glycol 6000, sucrose, magnesium carbonate, titanium dioxide, methylparaben, and polyvinyl alcohol.
LAB-0510-8.0 |
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
The recommended dose of EXEMESTANE in early and advanced breast cancer is one 25 mg tablet once daily after a meal.
•
adjuvant treatment of postmenopausal women with estrogen-receptor positive early breast cancer who have received two to three years of tamoxifen and are switched to EXEMESTANE for completion of a total of five consecutive years of adjuvant hormonal therapy.
•
the treatment of advanced breast cancer in postmenopausal women whose disease has progressed following tamoxifen therapy.
2.2 Dose Modifications
Concomitant use of strong CYP 3A4 inducers decreases exemestane exposure, For patients receiving EXEMESTANE with a strong CYP 3A4 inducer such as rifampicin or phenytoin, the recommended dose of EXEMESTANE is 50 mg once daily after a meal. [see Drug Interactions (7) and Clinical Pharmacology (12.3)]
Recommended Dose: One 25 mg tablet once daily after a meal (2.1).
DESCRIPTION SECTION
11 DESCRIPTION
EXEMESTANE tablets for oral administration contain 25 mg of exemestane, an irreversible, steroidal aromatase inactivator. Exemestane is chemically described as 6-methylenandrosta-1,4-diene-3,17-dione. Its molecular formula is C20H24O2 and its structural formula is as follows:
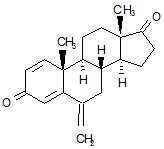
The active ingredient is a white to slightly yellow crystalline powder with a molecular weight of 296.41. Exemestane is freely soluble in N, N-dimethylformamide, soluble in methanol, and practically insoluble in water.
Each EXEMESTANE tablet contains the following inactive ingredients: mannitol, crospovidone, polysorbate 80, hypromellose, colloidal silicon dioxide, microcrystalline cellulose, sodium starch glycolate, magnesium stearate, simethicone, polyethylene glycol 6000, sucrose, magnesium carbonate, titanium dioxide, methylparaben, and polyvinyl alcohol.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Adjuvant Treatment in Early Breast Cancer
The Intergroup Exemestane Study 031 (IES) was a randomized, double-blind, multicenter, multinational study comparing exemestane (25 mg/day) vs. tamoxifen (20 or 30 mg/day) in postmenopausal women with early breast cancer. Patients who remained disease-free after receiving adjuvant tamoxifen therapy for 2 to 3 years were randomized to receive an additional 3 or 2 years of EXEMESTANE or tamoxifen to complete a total of 5 years of hormonal therapy.
The primary objective of the study was to determine whether, in terms of disease-free survival, it was more effective to switch to EXEMESTANE rather than continuing tamoxifen therapy for the remainder of five years. Disease- free survival was defined as the time from randomization to time of local or distant recurrence of breast cancer, contralateral invasive breast cancer, or death from any cause.
The secondary objectives were to compare the two regimens in terms of overall survival and long-term tolerability. Time to contralateral invasive breast cancer and distant recurrence-free survival were also evaluated.
A total of 4724 patients in the intent-to-treat (ITT) analysis were randomized to EXEMESTANE (exemestane tablets) 25 mg once daily (N = 2352) or to continue to receive tamoxifen once daily at the same dose received before randomization (N = 2372). Demographics and baseline tumor characteristics are presented in Table 5. Prior breast cancer therapy is summarized in Table 6.
Table 5. Demographic and Baseline Tumor Characteristics from the IES Study of Postmenopausal Women with Early Breast Cancer (ITT Population)|
Parameter |
Exemestane |
Tamoxifen |
|---|---|---|
| ||
|
Age (years): | ||
|
Median age (range) |
63.0 (38.0 – 96.0) |
63.0 (31.0 – 90.0) |
|
Race, n (%): | ||
|
Caucasian |
2315 (98.4) |
2333 (98.4) |
|
Hispanic |
13 (0.6) |
13 (0.5) |
|
Asian |
10 (0.4) |
9 (0.4) |
|
Black |
7 (0.3) |
10 (0.4) |
|
Other/not reported |
7 (0.3) |
7 (0.3) |
|
Nodal status, n (%): | ||
|
Negative |
1217 (51.7) |
1228 (51.8) |
|
Positive |
1051 (44.7) |
1044 (44.0) |
|
1–3 Positive nodes |
721 (30.7) |
708 (29.8) |
|
4–9 Positive nodes |
239 (10.2) |
244 (10.3) |
|
88 (3.7) |
86 (3.6) |
|
Not reported |
3 (0.1) |
6 (0.3) |
|
Unknown or missing |
84 (3.6) |
100 (4.2) |
|
Histologic type, n (%): | ||
|
Infiltrating ductal |
1777 (75.6) |
1830 (77.2) |
|
Infiltrating lobular |
341 (14.5) |
321 (13.5) |
|
Other |
231 (9.8) |
213 (9.0) |
|
Unknown or missing |
3 (0.1) |
8 (0.3) |
|
Receptor status***, n (%):** | ||
|
ER and PgR Positive |
1331 (56.6) |
1319 (55.6) |
|
ER Positive and PgR Negative/Unknown |
677 (28.8) |
692 (29.2) |
|
ER Unknown and PgR Positive†/Unknown |
288 (12.2) |
291 (12.3) |
|
ER Negative and PgR Positive |
6 (0.3) |
7 (0.3) |
|
ER Negative and PgR Negative/Unknown (none positive) |
48 (2.0) |
58 (2.4) |
|
Missing |
2 (0.1) |
5 (0.2) |
|
Tumor Size, n (%): | ||
|
≤ 0.5 cm |
58 (2.5) |
46 (1.9) |
|
315 (13.4) |
302 (12.7) |
|
1031 (43.8) |
1033 (43.5) |
|
833 (35.4) |
883 (37.2) |
|
62 (2.6) |
59 (2.5) |
|
Not reported |
53 (2.3) |
49 (2.1) |
|
Tumor Grade, n (%): | ||
|
G1 |
397 (16.9) |
393 (16.6) |
|
G2 |
977 (41.5) |
1007 (42.5) |
|
G3 |
454 (19.3) |
428 (18.0) |
|
G4 |
23 (1.0) |
19 (0.8) |
|
Unknown/Not Assessed/Not reported |
501 (21.3) |
525 (22.1) |
|
Parameter |
Exemestane |
Tamoxifen |
|---|---|---|
| ||
|
Type of surgery, n (%): | ||
|
Mastectomy |
1232 (52.4) |
1242 (52.4) |
|
Breast-conserving |
1116 (47.4) |
1123 (47.3) |
|
Unknown or missing |
4 (0.2) |
7 (0.3) |
|
Radiotherapy to the breast, n (%): | ||
|
Yes |
1524 (64.8) |
1523 (64.2) |
|
No |
824 (35.5) |
843 (35.5) |
|
Not reported |
4 (0.2) |
6 (0.3) |
|
Prior therapy, n (%): | ||
|
Chemotherapy |
774 (32.9) |
769 (32.4) |
|
Hormone replacement therapy |
567 (24.1) |
561 (23.7) |
|
Bisphosphonates |
43 (1.8) |
34 (1.4) |
|
Duration of tamoxifen therapy at randomization (months): | ||
|
Median (range) |
28.5 (15.8 – 52.2) |
28.4 (15.6 – 63.0) |
|
Tamoxifen dose, n (%): | ||
|
20 mg |
2270 (96.5) |
2287 (96.4) |
|
30 mg* |
78 (3.3) |
75 (3.2) |
|
Not reported |
4 (0.2) |
10 (0.4) |
After a median duration of therapy of 27 months and with a median follow-up of 34.5 months, 520 events were reported, 213 in the EXEMESTANE group and 307 in the tamoxifen group (Table 7).
Table 7. Primary Endpoint Events (ITT Population)|
Event |
First Events | |
|---|---|---|
|
Exemestane |
Tamoxifen | |
|
Loco-regional recurrence |
34 (1.45) |
45 (1.90) |
|
Distant recurrence |
126 (5.36) |
183 (7.72) |
|
Second primary – contralateral breast cancer |
7 (0.30) |
25 (1.05) |
|
Death – breast cancer |
1 (0.04) |
6 (0.25) |
|
Death – other reason |
41 (1.74) |
43 (1.81) |
|
Death – missing/unknown |
3 (0.13) |
5 (0.21) |
|
Ipsilateral breast cancer |
1 (0.04) |
0 |
|
Total number of events |
213 (9.06) |
307 (12.94) |
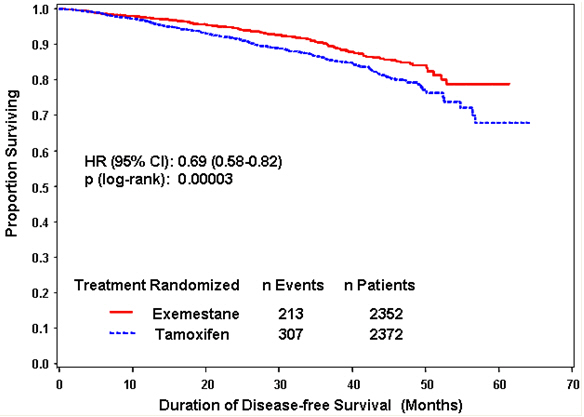
Disease-free survival in the intent-to-treat population was statistically significantly improved [Hazard Ratio (HR) = 0.69, 95% CI: 0.58, 0.82, P = 0.00003, Table 8, Figure 1] in the EXEMESTANE arm compared to the tamoxifen arm. In the hormone receptor-positive subpopulation representing about 85% of the trial patients, disease-free survival was also statistically significantly improved (HR = 0.65, 95% CI: 0.53, 0.79, P = 0.00001) in the EXEMESTANE arm compared to the tamoxifen arm. Consistent results were observed in the subgroups of patients with node negative or positive disease, and patients who had or had not received prior chemotherapy.
An overall survival update at 119 months median follow-up showed no significant difference between the two groups, with 467 deaths (19.9%) occurring in the EXEMESTANE group and 510 deaths (21.5%) in the tamoxifen group.
Table 8. Efficacy Results from the IES Study in Postmenopausal Women with Early Breast Cancer
| ||
|
ITT Population |
Hazard Ratio |
p-value |
|
Disease-free survival |
0.69 (0.58–0.82) |
0.00003 |
|
Time to contralateral breast cancer |
0.32 (0.15–0.72) |
0.00340 |
|
Distant recurrence-free survival |
0.74 (0.62–0.90) |
0.00207 |
|
Overall survival |
0.91 (0.81–1.04) |
0.16* |
|
ER and/or PgR positive | ||
|
Disease-free survival |
0.65 (0.53–0.79) |
0.00001 |
|
Time to contralateral breast cancer |
0.22 (0.08–0.57) |
0.00069 |
|
Distant recurrence-free survival |
0.73 (0.59–0.90) |
0.00367 |
|
Overall survival |
0.89 (0.78–1.02) |
0.09065* |
Figure 1. Disease-Free Survival in the IES Study of Postmenopausal Women with Early Breast Cancer (ITT Population)
14.2 Treatment of Advanced Breast Cancer
Exemestane 25 mg administered once daily was evaluated in a randomized double- blind, multicenter, multinational comparative study and in two multicenter single-arm studies of postmenopausal women with advanced breast cancer who had disease progression after treatment with tamoxifen for metastatic disease or as adjuvant therapy. Some patients also have received prior cytotoxic therapy, either as adjuvant treatment or for metastatic disease.
The primary purpose of the three studies was evaluation of objective response rate (complete response [CR] and partial response [PR]). Time to tumor progression and overall survival were also assessed in the comparative trial. Response rates were assessed based on World Health Organization (WHO) criteria, and in the comparative study, were submitted to an external review committee that was blinded to patient treatment. In the comparative study, 769 patients were randomized to receive EXEMESTANE tablets 25 mg once daily (N = 366) or megestrol acetate 40 mg four times daily (N = 403). Demographics and baseline characteristics are presented in Table 9.
Table 9. Demographics and Baseline Characteristics from the Comparative Study of Postmenopausal Women with Advanced Breast Cancer Whose Disease Had Progressed after Tamoxifen Therapy|
Parameter |
EXEMESTANE |
Megestrol Acetate |
|---|---|---|
|
Median Age (range) |
65 (35–89) |
65 (30–91) |
|
ECOG Performance Status | ||
|
0 |
167 (46%) |
187 (46%) |
|
1 |
162 (44%) |
172 (43%) |
|
2 |
34 (9%) |
42 (10%) |
|
Receptor Status | ||
|
ER and/or PgR + |
246 (67%) |
274 (68%) |
|
ER and PgR unknown |
116 (32%) |
128 (32%) |
|
Responders to prior tamoxifen |
68 (19%) |
85 (21%) |
|
NE for response to prior tamoxifen |
46 (13%) |
41 (10%) |
|
Site of Metastasis | ||
|
Visceral ± other sites |
207 (57%) |
239 (59%) |
|
Bone only |
61 (17%) |
73 (18%) |
|
Soft tissue only |
54 (15%) |
51 (13%) |
|
Bone & soft tissue |
43 (12%) |
38 (9%) |
|
Measurable Disease |
287 (78%) |
314 (78%) |
|
Prior Tamoxifen Therapy | ||
|
Adjuvant or Neoadjuvant |
145 (40%) |
152 (38%) |
|
Advanced Disease, Outcome | ||
|
CR, PR, or SD ≥ 6 months |
179 (49%) |
210 (52%) |
|
SD < 6 months, PD or NE |
42 (12%) |
41 (10%) |
|
Prior Chemotherapy | ||
|
For advanced disease ± adjuvant |
58 (16%) |
67 (17%) |
|
Adjuvant only |
104 (28%) |
108 (27%) |
|
No chemotherapy |
203 (56%) |
226 (56%) |
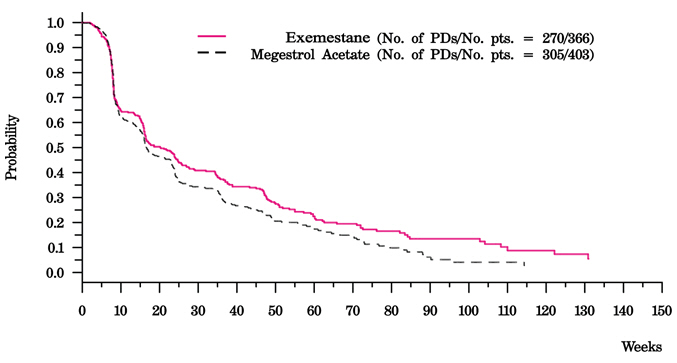
The efficacy results from the comparative study are shown in Table 10. The objective response rates observed in the two treatment arms showed that EXEMESTANE was not different from megestrol acetate. Response rates for EXEMESTANE from the two single-arm trials were 23.4% and 28.1%.
Table 10. Efficacy Results from the Comparative Study of Postmenopausal Women with Advanced Breast Cancer Whose Disease Had Progressed after Tamoxifen Therapy|
Response Characteristics |
EXEMESTANE |
Megestrol Acetate |
|---|---|---|
|
Abbreviations: CR = complete response, PR = partial response, SD = stable disease (no change), TTP = time to tumor progression, C.I. = confidence interval, MA = megestrol acetate, AR = EXEMESTANE | ||
|
Objective Response Rate = CR + PR (%) |
15.0 |
12.4 |
|
Difference in Response Rate (AR-MA) |
2.6 | |
|
95% C.I. |
7.5, -2.3 | |
|
CR (%) |
2.2 |
1.2 |
|
PR (%) |
12.8 |
11.2 |
|
SD ≥ 24 Weeks (%) |
21.3 |
21.1 |
|
Median Duration of Response (weeks) |
76.1 |
71.0 |
|
Median TTP (weeks) |
20.3 |
16.6 |
|
Hazard Ratio (AR-MA) |
0.84 |
There were too few deaths occurring across treatment groups to draw conclusions on overall survival differences. The Kaplan-Meier curve for time to tumor progression in the comparative study is shown in Figure 2.
|
Figure 2. Time to Tumor Progression in the Comparative Study of Postmenopausal Women With Advanced Breast Cancer Whose Disease Had Progressed After Tamoxifen Therapy |
|---|
|
|
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Bone Effects
Advise patients that EXEMESTANE lowers the level of estrogen in the body. This may lead to reduction in bone mineral density (BMD) over time. The lower the BMD, the greater the risk of osteoporosis and fracture [see Warnings and Precautions (5.1)].
Other Estrogen-Containing Agents
Advise patients that they should not take estrogen-containing agents while they are taking EXEMESTANE as these could interfere with its pharmacologic action [see Warnings and Precautions (5.3)].
Use in Premenopausal Women
Advise patients that EXEMESTANE is not for use for the treatment of breast cancer in premenopausal women [see Warnings and Precautions (5.5)].
Embryo-Fetal Toxicity
Advise pregnant women and females of reproductive potential that exposure during pregnancy or within 1 month prior to conception can result in fetal harm. Advise females to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.6) and Use in Specific Populations (8.1)].
Advise females of reproductive potential to use effective contraception while taking EXEMESTANE and for 1 month after the last dose [see Use in Specific Populations (8.3)].
Lactation
Advise women not to breastfeed during treatment with EXEMESTANE and for 1 month after the last dose [see Use in Specific Populations (8.2)].