SYZ CEPHALANDRA
BM HOMEOPATHIC LIQUID SYZ CEPHALANDRA
3b3a3776-96c0-f876-e063-6394a90aaec8
HUMAN OTC DRUG LABEL
Jul 31, 2025
BM Private Limited
DUNS: 645599762
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
acid phos, cephalandra ind, gynmema syl, momordica charantia, syz jambol
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
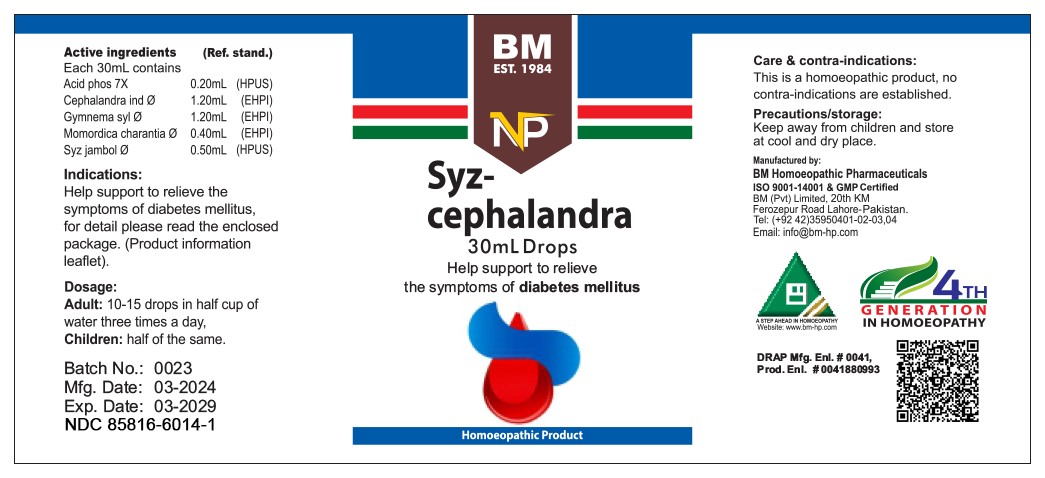
INDICATIONS & USAGE SECTION
Help support to relieve the symptoms of diabetes mellitus, for detail page please read the enclosed package (product information leaflet).
CONTRAINDICATIONS SECTION
Care & contra-indications:
This is a homoeopathic remedy, no contra-indications are established.
OTC - PURPOSE SECTION
Help support to relieve the symptoms of diabetes mellitus, for detail page please read the enclosed package (product information leaflet).
WARNINGS SECTION
Precautions/storage:
Keep away from children and store at cool and dry place.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Precautions/storage:
Keep away from children and store at cool and dry place.
DOSAGE & ADMINISTRATION SECTION
Dosage:
Adult:10-15 drops in half cup of water three times a day
Children: half of the same
OTC - ACTIVE INGREDIENT SECTION
acid phos, cephalandra ind, gynmema syl, momordica charantia, syz jambol
INACTIVE INGREDIENT SECTION
ALCOHOL
