Nystatin
NYSTATIN CREAM USP
840eebe8-5de2-40c5-acfd-03a98b2e790e
HUMAN PRESCRIPTION DRUG LABEL
Sep 23, 2025
Bryant Ranch Prepack
DUNS: 171714327
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Nystatin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Nystatin Cream USP (100,000 USP Nystatin Units)
SPL UNCLASSIFIED SECTION
Manufactured By Padagis
Yeruham, Israel
Distributed By Padagis
Allegan, MI 49010
www.padagis.com
Rev 12-22
68R00 RC J1
DESCRIPTION SECTION
DESCRIPTION
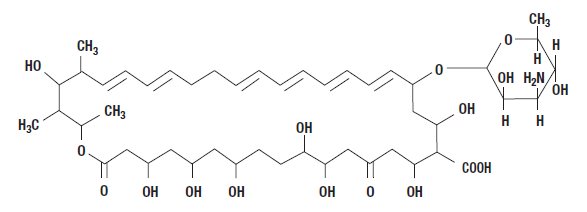
Nystatin is a polyene antifungal antibiotic obtained from Streptomyces noursei. The molecular formula is C47H75NO17, and the molecular weight is 926.13.
Structural formula:
Nystatin Cream USP is for dermatologic use.
Nystatin Cream USP for topical use, contains 100,000 USP nystatin units per gram. Inactive ingredients: emulsifying wax, glycerin, isopropyl myristate, lactic acid, purified water, sodium hydroxide, and sorbic acid.
CLINICAL PHARMACOLOGY SECTION
CLINICAL PHARMACOLOGY
Pharmacokinetics
Nystatin Cream USP is not absorbed from intact skin or mucous membrane.
Microbiology
Nystatin is an antibiotic which is both fungistatic and fungicidal in vitro against a wide variety of yeasts and yeast-like fungi, including Candida albicans, C. parapsilosis, C. tropicalis, C. guilliermondi, C. pseudotropicalis, C. krusei, Torulopsis glabrata, Tricophyton rubrum, T. mentagrophytes.
Nystatin acts by binding to sterols in the cell membrane of susceptible species resulting in a change in membrane permeability and the subsequent leakage of intracellular components. On repeated subculturing with increasing levels of nystatin, Candida albicans does not develop resistance to nystatin. Generally, resistance to nystatin does not develop during therapy. However, other species of Candida(C. tropicalis, C. guilliermondi, C. krusei, and C. stellatoides) become quite resistant on treatment with nystatin and simultaneously become cross resistant to amphotericin as well. This resistance is lost when the antibiotic is removed.
Nystatin exhibits no appreciable activity against bacteria, protozoa, or viruses.
PRECAUTIONS SECTION
PRECAUTIONS
General -
Nystatin Cream USP should not be used for the treatment of systemic, oral, intravaginal or ophthalmic infections.
If irritation or sensitization develops, treatment should be discontinued and appropriate measures taken as indicated. It is recommended that KOH smears, cultures, or other diagnostic methods be used to confirm the diagnosis of cutaneous or mucocutaneous candidiasis and to rule out infection caused by other pathogens.
Information for Patients -
Patients using this medication should receive the following information and instructions:
1. The patient should be instructed to use this medication as directed (including the replacement of missed doses). This medication is not for any disorder other than that for which it is prescribed.
2. Even if symptomatic relief occurs within the first few days of treatment, the patient should be advised not to interrupt or discontinue therapy until the prescribed course of treatment is completed.
3. If symptoms of irritation develop, the patient should be advised to notify the physician promptly.
Laboratory Tests -
If there is a lack of therapeutic response, KOH smears, cultures, or other diagnostic methods should be repeated.
Carcinogenesis, Mutagenesis, Impairment of Fertility -
No long-term animal studies have been performed to evaluate the carcinogenic potential of Nystatin. No studies have been performed to determine the mutagenicity of Nystatin or its effects on male or female fertility.
Pregnancy: Teratogenic Effects: Category C -
Animal reproduction studies have not been conducted with any Nystatin topical preparation. It also is not known whether these preparations can cause fetal harm when used by a pregnant woman or can affect reproductive capacity. Nystatin topical preparations should be prescribed for a pregnant woman only if the potential benefit to the mother outweighs the potential risk to the fetus.
Nursing Mothers -
It is not known whether Nystatin is excreted in human milk. Caution should be exercised when nystatin is prescribed for a nursing woman.
Pediatric Use -
Safety and effectiveness have been established in the pediatric population from birth to 16 years (seeDOSAGE AND ADMINISTRATION).
Geriatric Use -
Clinical studies with nystatin cream did not include sufficient numbers of subjects aged 65 years and older to determine whether they respond differently than younger subjects. Other reported clinical experience has not identified differences in responses between elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
HOW SUPPLIED SECTION
HOW SUPPLIED
Nystatin Cream USP (100,000 USP Nystatin Units per gram) is a yellow cream available as follows:
15 g tube (NDC 63629-8694-1)
Storage
Store at 20-25°C (68-77°F) [see USP Controlled Room Temperature].
Repackaged/Relabeled by:
Bryant Ranch Prepack, Inc.
Burbank, CA 91504
