Manufacturing Establishments (1)
Baxter Healthcare Company
194684502
Products (3)
Nitroglycerin In Dextrose
0338-1051
NDA019970
NDA (C73594)
INTRAVENOUS
August 22, 2016
Nitroglycerin In Dextrose
0338-1047
NDA019970
NDA (C73594)
INTRAVENOUS
August 22, 2016
Nitroglycerin In Dextrose
0338-1049
NDA019970
NDA (C73594)
INTRAVENOUS
August 22, 2016
Drug Labeling Information
DESCRIPTION SECTION
DESCRIPTION
Nitroglycerin is 1,2,3-propanetriol trinitrate, an organic nitrate whose structural formula is
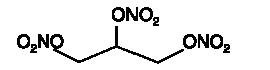
whose empiric formula is C3H5N3O9, and whose molecular weight is 227.09. The organic nitrates are vasodilators, active on both arteries and veins.
Dextrose (Dextrose Hydrous, USP) is D-glucose monohydrate, a hexose sugar whose structural formula is
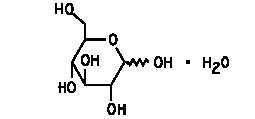
whose empiric formula is C6H12O6 • H2O, and whose molecular weight is 198.17.
Dextrose is derived from corn.
Nitroglycerin in 5% Dextrose Injection is a sterile, nonpyrogenic solution of nitroglycerin and dextrose in water for injection. The solution is clear and practically colorless. Each 100 mL contains 10 mg, 20 mg, or 40 mg nitroglycerin (added as Diluted Nitroglycerin, USP with propylene glycol); 5 g Dextrose Hydrous, USP; 0.84 mL Alcohol, USP (added as a dissolution aid); and 105 mg Citric Acid Hydrous, USP (added as a buffer). The pH of the solution is adjusted with sodium hydroxide and, if necessary, hydrochloric acid.
Although dry nitroglycerin is explosive, nitroglycerin in 5% dextrose is not.
Composition, osmolarity and pH are given in Table 1.
| ||||
|
Table 1 |
Composition |
*Osmolarity |
pH | |
|
Nitroglycerin |
Dextrose | |||
|
25 mg Nitroglycerin |
100 |
50 |
428 |
4.0 (3.0 to 5.0) |
|
50 mg Nitroglycerin |
200 |
50 |
440 |
4.0 (3.0 to 5.0) |
|
100 mg Nitroglycerin |
400 |
50 |
465 |
4.0 (3.0 to 5.0) |
