Nebivolol
These highlights do not include all the information needed to use NEBIVOLOL TABLETS safely and effectively. See full prescribing information for NEBIVOLOL TABLETS NEBIVOLOL tablets, for oral use Initial U.S. Approval: 2007
b6c5abdd-bc2d-4ed6-8ca2-8a94c406ed0e
HUMAN PRESCRIPTION DRUG LABEL
Apr 8, 2025
Macleods Pharmaceuticals Limited
DUNS: 862128535
Products 4
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Nebivolol
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
Nebivolol
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Nebivolol
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
Nebivolol
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
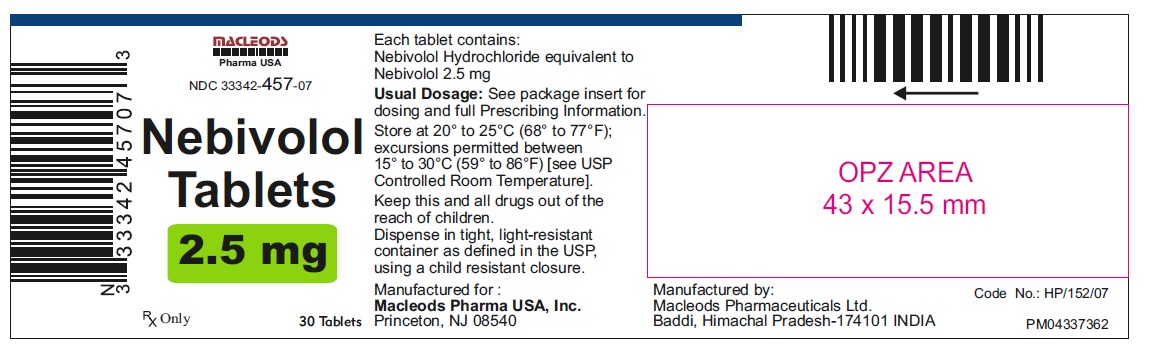
Nebivolol Tablets, 2.5 mg
30's Container Pack
NDC: 33342-457-07
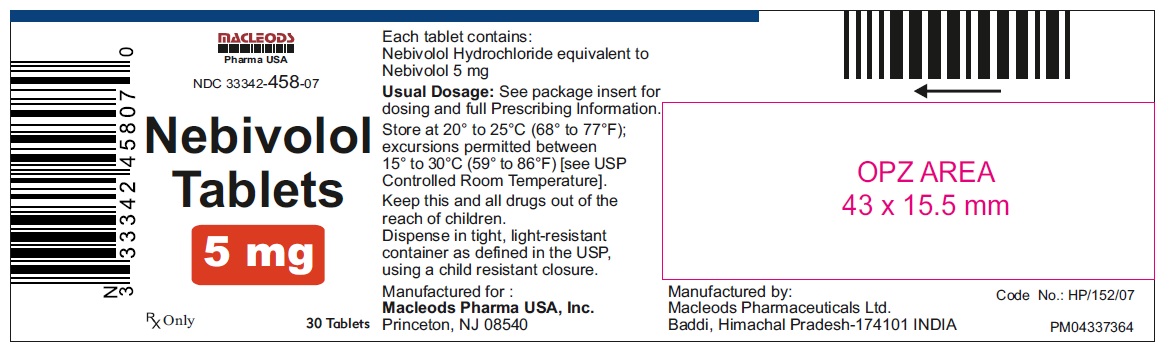
Nebivolol Tablets, 5 mg
30's Container Pack
NDC: 33342-458-07
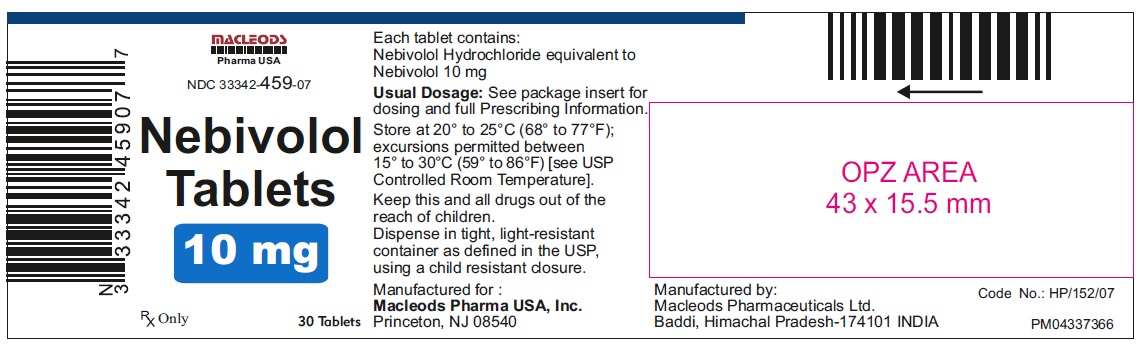
Nebivolol Tablets, 10 mg
30's Container Pack
NDC: 33342-459-07
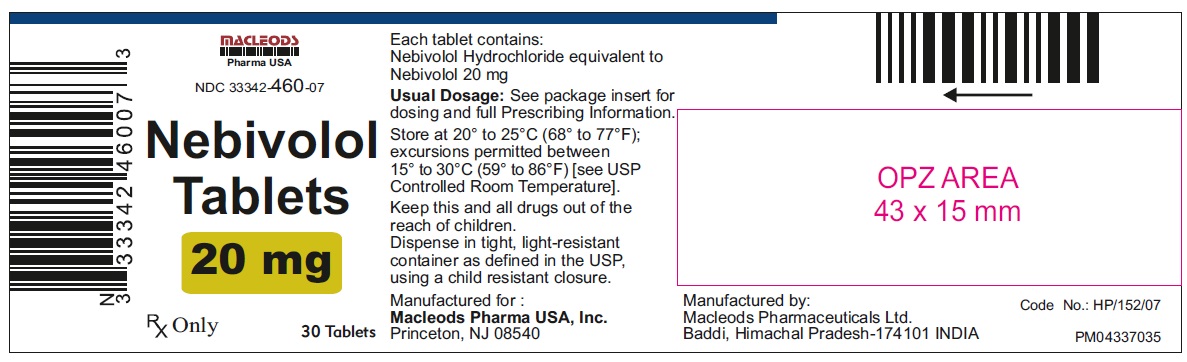
Nebivolol Tablets, 20 mg
30's Container Pack
NDC: 33342-460-07
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
1.1 Hypertension
Nebivolol tablets are indicated for the treatment of hypertension, to lower blood pressure [see Clinical Studies (14.1)]. Nebivolol tablets may be used alone or in combination with other antihypertensive agents [see Drug Interactions (7)].
Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes, including the class to which this drug principally belongs. There are no controlled trials demonstrating risk reduction with nebivolol tablets.
Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program’s Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC).
Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly.
Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal.
Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy.
Nebivolol tablets are a beta-adrenergic blocking agent indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. (1.1)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
Nebivolol tablets are contraindicated in the following conditions:
• Severe bradycardia
• Heart block greater than first degree
• Patients with cardiogenic shock
• Decompensated cardiac failure
• Sick sinus syndrome (unless a permanent pacemaker is in place)
• Patients with severe hepatic impairment (Child-Pugh >B)
• Patients who are hypersensitive to any component of this product.
• Severe bradycardia (4)
• Heart block greater than first degree (4)
• Patients with cardiogenic shock (4)
• Decompensated cardiac failure (4)
• Sick sinus syndrome (unless a permanent pacemaker is in place) (4)
• Patients with severe hepatic impairment (Child-Pugh >B) (4)
• Hypersensitive to any component of this product (4)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Abrupt Cessation of Therapy
Do not abruptly discontinue nebivolol tablets therapy in patients with coronary artery disease. Severe exacerbation of angina, myocardial infarction and ventricular arrhythmias have been reported in patients with coronary artery disease following the abrupt discontinuation of therapy with β-blockers. Myocardial infarction and ventricular arrhythmias may occur with or without preceding exacerbation of the angina pectoris. Caution patients without overt coronary artery disease against interruption or abrupt discontinuation of therapy. As with other β-blockers, when discontinuation of nebivolol tablets are planned, carefully observe and advise patients to minimize physical activity. Taper nebivolol tablets over 1 to 2 weeks when possible. If the angina worsens or acute coronary insufficiency develops, re- start nebivolol tablets promptly, at least temporarily.
5.2 Angina and Acute Myocardial Infarction
Nebivolol tablets were not studied in patients with angina pectoris or who had a recent MI.
5.3 Bronchospastic Diseases
In general, patients with bronchospastic diseases should not receive β-blockers.
5.4 Anesthesia and Major Surgery
Because beta-blocker withdrawal has been associated with an increased risk of
MI and chest pain, patients already on beta-blockers should generally continue
treatment throughout the perioperative period. If nebivolol tablets are to be
continued perioperatively, monitor patients closely when anesthetic agents
which depress myocardial function, such as ether, cyclopropane, and
trichloroethylene, are used. If β-blocking therapy is withdrawn prior to major
surgery, the impaired ability of the heart to respond to reflex adrenergic
stimuli may augment the risks of general anesthesia and surgical procedures.
The β-blocking effects of nebivolol tablets can be reversed by β-agonists,
e.g., dobutamine or isoproterenol. However, such patients may be subject to
protracted severe hypotension. Additionally, difficulty in restarting and
maintaining the heartbeat has been reported with β-blockers.
5.5 Hypoglycemia
Beta-blockers may prevent early warning signs of hypoglycemia, such as tachycardia, and increase the risk for severe or prolonged hypoglycemia at anytime during treatment, especially in patients with diabetes mellitus or children and patients who are fasting (i.e., surgery, not eating regularly, or arevomiting). If severe hypoglycemia occurs, patients should be instructed to seek emergency treatment.
5.6 Thyrotoxicosis
β-blockers may mask clinical signs of hyperthyroidism, such as tachycardia. Abrupt withdrawal of β-blockers may be followed by an exacerbation of the symptoms of hyperthyroidism or may precipitate a thyroid storm.
5.7 Peripheral Vascular Disease
β-blockers can precipitate or aggravate symptoms of arterial insufficiency in patients with peripheral vascular disease.
5.8 Non-dihydropyridine Calcium Channel Blockers
Because of significant negative inotropic and chronotropic effects in patients treated with β-blockers and calcium channel blockers of the verapamil and diltiazem type, monitor the ECG and blood pressure in patients treated concomitantly with these agents.
5.9 Use with CYP2D6 Inhibitors
Nebivolol exposure increases with inhibition of CYP2D6 [see Drug Interactions (7)]. The dose of nebivolol tablets may need to be reduced.
5.10 Impaired Renal Function
Renal clearance of nebivolol is decreased in patients with severe renal impairment. Nebivolol tablets have not been studied in patients receiving dialysis [see Clinical Pharmacology (12.4) and Dosage and Administration (2.1)].
5.11 Impaired Hepatic Function
Metabolism of nebivolol is decreased in patients with moderate hepatic impairment. Nebivolol tablets have not been studied in patients with severe hepatic impairment [see Clinical Pharmacology (12.4) and Dosage and Administration (2.1)].
5.12 Risk of Anaphylactic Reactions
While taking β-blockers, patients with a history of severe anaphylactic reactions to a variety of allergens may be more reactive to repeated accidental, diagnostic, or therapeutic challenge. Such patients may be unresponsive to the usual doses of epinephrine used to treat allergic reactions.
5.13 Pheochromocytoma
In patients with known or suspected pheochromocytoma, initiate an α-blocker prior to the use of any β-blocker.
• Acute exacerbation of coronary artery disease upon cessation of therapy: Do
not abruptly discontinue. (5.1)
• Diabetes: May mask symptoms of hypoglycemia and alter glucose levels;
monitor (5.5)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
Nebivolol tablets have been evaluated for safety in patients with hypertension
and in patients with heart failure. The observed adverse reaction profile was
consistent with the pharmacology of the drug and the health status of the
patients in the clinical trials. Adverse reactions reported for each of these
patient populations are provided below. Excluded are adverse reactions
considered too general to be informative and those not reasonably associated
with the use of the drug because they were associated with the condition being
treated or are very common in the treated population.
The data described below reflect worldwide clinical trial exposure to
nebivolol tablets in 6545 patients, including 5038 patients treated for
hypertension and the remaining 1507 subjects treated for other cardiovascular
diseases. Doses ranged from 0.5 mg to 40 mg. Patients received nebivolol
tablets for up to 24 months, with over 1900 patients treated for at least 6
months, and approximately 1300 patients for more than one year.
HYPERTENSION: In placebo-controlled clinical trials comparing nebivolol tablets with placebo, discontinuation of therapy due to adverse reactions was reported in 2.8% of patients treated with nebivolol and 2.2% of patients given placebo. The most common adverse reactions that led to discontinuation of nebivolol tablets were headache (0.4%), nausea (0.2%) and bradycardia (0.2%).
Table 1 lists treatment-emergent adverse reactions that were reported in
three 12-week, placebo-controlled monotherapy trials involving 1597
hypertensive patients treated with either 5 mg, 10 mg, or 20-40 mg of
nebivolol tablets and 205 patients given placebo and for which the rate of
occurrence was at least 1% of patients treated with nebivolol and greater than
the rate for those treated with placebo in at least one dose group.
**Table 1.**Treatment-Emergent Adverse Reactions with an Incidence (over 6
weeks) ≥ 1% in nebivolol tablets-Treated Patients and at a Higher Frequency
than Placebo-Treated Patients
|
System Organ Class – Preferred Term |
Placebo (n = 205) (%) |
Nebivolol 5 mg (n = 459) (%) |
Nebivolol 10 mg (n = 461) (%) |
Nebivolol 20-40 mg (n = 677) (%) |
|
Cardiac Disorders | ||||
|
Bradycardia |
0 |
0 |
0 |
1 |
|
Gastrointestinal Disorders | ||||
|
Diarrhea |
2 |
2 |
2 |
3 |
|
Nausea |
0 |
1 |
3 |
2 |
|
General Disorders | ||||
|
Fatigue |
1 |
2 |
2 |
5 |
|
Chest pain |
0 |
0 |
1 |
1 |
|
Peripheral edema |
0 |
1 |
1 |
1 |
|
Nervous System Disorders | ||||
|
Headache |
6 |
9 |
6 |
7 |
|
Dizziness |
2 |
2 |
3 |
4 |
|
Psychiatric Disorders | ||||
|
Insomnia |
0 |
1 |
1 |
1 |
|
Respiratory Disorders | ||||
|
Dyspnea |
0 |
0 |
1 |
1 |
|
Skin and subcutaneous Tissue Disorders | ||||
|
Rash |
0 |
0 |
1 |
1 |
Listed below are other reported adverse reactions with an incidence of at least 1% in the more than 4300 patients treated with nebivolol tablets in controlled or open-label trials except for those already appearing in** Table 1**, terms too general to be informative, minor symptoms, or adverse reactions unlikely to be attributable to drug because they are common in the population. These adverse reactions were in most cases observed at a similar frequency in placebo-treated patients in the controlled studies.
Body as a Whole: asthenia.
Gastrointestinal System Disorders: abdominal pain
Metabolic and Nutritional Disorders: hypercholesterolemia
Nervous System Disorders: paraesthesia
6.2 Laboratory Abnormalities
In controlled monotherapy trials of hypertensive patients, nebivolol tablets was associated with an increase in BUN, uric acid, triglycerides and a decrease in HDL cholesterol and platelet count.
6.3 Postmarketing Experience
The following adverse reactions have been identified from spontaneous reports of nebivolol tablets received worldwide and have not been listed elsewhere. These adverse reactions have been chosen for inclusion due to a combination of seriousness, frequency of reporting or potential causal connection to nebivolol tablets. Adverse reactions common in the population have generally been omitted. Because these adverse reactions were reported voluntarily from a population of uncertain size, it is not possible to estimate their frequency or establish a causal relationship to nebivolol tablets exposure: abnormal hepatic function (including increased AST, ALT and bilirubin), acute pulmonary edema, acute renal failure, atrioventricular block (both second and third degree), bronchospasm, erectile dysfunction, hypersensitivity (including urticaria, allergic vasculitis and rare reports of angioedema), hypotension, myocardial infarction, pruritus, psoriasis, Raynaud’s phenomenon, peripheral ischemia/claudication, somnolence, syncope, thrombocytopenia, various rashes and skin disorders, vertigo, and vomiting.
Most common adverse reactions (6.1):
• Headache, fatigue
To report SUSPECTED ADVERSE REACTIONS, Contact Macleods Pharma USA, Inc., at 1-888-943-3210 or 1-855-926-3384o r FDA at 1-800-FDA-1088 or www.fda.gov/medwatch**.**
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 CYP2D6 Inhibitors
Use caution when nebivolol tablets are co-administered with CYP2D6 inhibitors (quinidine, propafenone, fluoxetine, paroxetine, etc.) [see Clinical Pharmacology (12.5)].
7.2 Hypotensive Agents
Do not use nebivolol tablets with other β-blockers. Closely monitor patients receiving catecholamine-depleting drugs, such as reserpine or guanethidine, because the added β-blocking action of nebivolol tablets may produce excessive reduction of sympathetic activity. In patients who are receiving nebivolol tablets and clonidine, discontinue nebivolol tablets for several days before the gradual tapering of clonidine.
7.3 Digitalis Glycosides
Both digitalis glycosides and β-blockers slow atrioventricular conduction and decrease heart rate. Concomitant use can increase the risk of bradycardia.
7.4 Calcium Channel Blockers
Nebivolol tablets can exacerbate the effects of myocardial depressants or inhibitors of AV conduction, such as certain calcium antagonists (particularly of the phenylalkylamine [verapamil] and benzothiazepine [diltiazem] classes), or antiarrhythmic agents, such as disopyramide.
• CYP2D6 enzyme inhibitors may increase nebivolol levels. (7.1)
• Reserpine or clonidine may produce excessive reduction of sympathetic
activity. (7.2)
• Both digitalis glycosides and β-blockers slow atrioventricular conduction
and decrease heart rate. Concomitant use can increase the risk of bradycardia.
(7.3)
• Verapamil- or diltiazem-type calcium channel blockers may cause excessive
reductions in heart rate, blood pressure, and cardiac contractility. (7.4)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data regarding use of nebivolol tablets in pregnant women are
insufficient to determine whether there are drug-associated risks of adverse
developmental outcomes. There are risks to the mother and fetus associated
with poorly controlled hypertension in pregnancy. The use of beta blockers
during the third trimester of pregnancy may increase the risk of hypotension,
bradycardia, hypoglycemia, and respiratory depression in the neonate [see Clinical Considerations]. Oral administration of nebivolol to pregnant rats
during organogenesis resulted in embryofetal and perinatal lethality at doses
approximately equivalent to the maximum recommended human dose (MRHD).
The estimated background risk of major birth defects and miscarriage for the
indicated population is unknown. In the U.S. general population, the estimated
background risk of major birth defects and miscarriage in clinically
recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Hypertension in pregnancy increases the maternal risk for pre-eclampsia,
gestational diabetes, premature delivery, and delivery complications (e.g.,
need for cesarean section, and post-partum hemorrhage). Hypertension increases
the fetal risk for intrauterine growth restriction and intrauterine death.
Pregnant women with hypertension should be carefully monitored and managed
accordingly.
Fetal/Neonatal adverse reactions
Neonates of women with hypertension, who are treated with beta-blockers during
the third trimester of pregnancy, may be at increased risk for hypotension,
bradycardia, hypoglycemia, and respiratory depression. Observe newborns for
symptoms of hypotension, bradycardia, hypoglycemia and respiratory depression
and manage accordingly.
Data
Animal Data
Nebivolol was shown to increase embryo-fetal and perinatal lethality in rats
at approximately 1.2 times the MRHD or 40 mg/day on a mg/m2 basis. Decreased
pup body weights occurred at 1.25 and 2.5 mg/kg in rats, when exposed during
the perinatal period (late gestation, parturition and lactation). At 5 mg/kg
and higher doses (1.2 times the MRHD), prolonged gestation, dystocia and
reduced maternal care were produced with corresponding increases in late fetal
deaths and stillbirths and decreased birth weight, live litter size and pup
survival. These events occurred only when nebivolol was given during the
perinatal period (late gestation, parturition and lactation). Insufficient
numbers of pups survived at 5 mg/kg to evaluate the offspring for reproductive
performance.
In studies in which pregnant rats were given nebivolol during organogenesis,
reduced fetal body weights were observed at maternally toxic doses of 20 and
40 mg/kg/day (5 and 10 times the MRHD), and small reversible delays in sternal
and thoracic ossification associated with the reduced fetal body weights and a
small increase in resorption occurred at 40 mg/kg/day (10 times the MRHD).
No adverse effects on embryo-fetal viability, sex, weight or morphology were
observed in studies in which nebivolol was given to pregnant rabbits at doses
as high as 20 mg/kg/day (10 times the MRHD).
8.2 Lactation
Risk Summary
There is no information regarding the presence of nebivolol in human milk, the
effects on the breastfed infant, or the effects on milk production. Nebivolol
is present in rat milk [see Data]. Because of the potential for β-blockers to
produce serious adverse reactions in nursing infants, especially bradycardia,
nebivolol tablets are not recommended during nursing.
Data
In lactating rats, maximum milk levels of unchanged nebivolol were observed at
4 hours after single and repeat doses of 2.5 mg/kg/day. The daily dose (mg/kg
body weight) ingested by a rat pup is 0.3% of the dam dose for unchanged
nebivolol.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established. Pediatric studies in ages newborn to 18 years old have not been conducted because of incomplete characterization of developmental toxicity and possible adverse effects on long-term fertility [see Nonclinical Toxicology (13.1)].
Juvenile Animal Toxicity Data
Daily oral doses of nebivolol to juvenile rats from post-natal day 14 to post-
natal day 27 showed sudden unexplained death at exposures equal to those in
human poor metabolizers given a single dose of 10 mg. No mortality was seen at
half the adult human exposure.
In surviving rats, cardiomyopathy was seen at exposures greater than or equal to the human exposure. Male rat pups exposed to twice the human exposure showed decreases in total sperm count as well as decreases in the total and percentage of motile sperm.
8.5 Geriatric Use
Of the 2800 patients in the U.S. sponsored placebo-controlled clinical hypertension studies, 478 patients were 65 years of age or older. No overall differences in efficacy or in the incidence of adverse events were observed between older and younger patients.
8.6 Heart Failure
In a placebo-controlled trial of 2128 patients (1067 nebivolol tablets, 1061 placebo) over 70 years of age with chronic heart failure receiving a maximum dose of 10 mg per day for a median of 20 months, no worsening of heart failure was reported with nebivolol compared to placebo. However, if heart failure worsens consider discontinuation of nebivolol tablets.
• Lactation: Breastfeeding is not recommended. (8.2)
OVERDOSAGE SECTION
10 OVERDOSAGE
In clinical trials and worldwide postmarketing experience there were reports
of nebivolol tablets overdose. The most common signs and symptoms associated
with nebivolol tablets overdosage are bradycardia and hypotension. Other
important adverse reactions reported with nebivolol tablets overdose include
cardiac failure, dizziness, hypoglycemia, fatigue and vomiting. Other adverse
reactions associated with β-blocker overdose include bronchospasm and heart
block.
The largest known ingestion of nebivolol tablets worldwide involved a patient
who ingested up to 500 mg of nebivolol tablets along with several 100 mg
tablets of acetylsalicylic acid in a suicide attempt. The patient experienced
hyperhydrosis, pallor, depressed level of consciousness, hypokinesia,
hypotension, sinus bradycardia, hypoglycemia, hypokalemia, respiratory failure
and vomiting. The patient recovered.
Because of extensive drug binding to plasma proteins, hemodialysis is not
expected to enhance nebivolol clearance.
If overdose occurs, provide general supportive and specific symptomatic
treatment. Based on expected pharmacologic actions and recommendations for
other β-blockers, consider the following general measures, including stopping
nebivolol tablets, when clinically warranted:
Bradycardia: Administer IV atropine. If the response is inadequate,
isoproterenol or another agent with positive chronotropic properties may be
given cautiously. Under some circumstances, transthoracic or transvenous
pacemaker placement may be necessary.
Hypotension: Administer IV fluids and vasopressors. Intravenous glucagon may
be useful.
Heart Block (second or third degree): Monitor and treat with isoproterenol
infusion. Under some circumstances, transthoracic or transvenous pacemaker
placement may be necessary.
Congestive Heart Failure: Initiate therapy with digitalis glycoside and
diuretics. In certain cases, consider the use of inotropic and vasodilating
agents.
Bronchospasm: Administer bronchodilator therapy such as a short acting inhaled
β2-agonist and/or aminophylline.
Hypoglycemia: Administer IV glucose. Repeated doses of IV glucose or possibly
glucagon may be required.
Supportive measures should continue until clinical stability is achieved. The
half-life of low doses of nebivolol is 12-19 hours.
Call the National Poison Control Center (800-222-1222) for the most current
information on β-blocker overdose treatment.
DESCRIPTION SECTION
11 DESCRIPTION
The chemical name for the active ingredient in nebivolol tablets (nebivolol) tablets is (1RS,1’RS)-1,1’-[(2RS,2’SR)bis(6-fluoro-3,4-dihydro-2H-1-benzopyran-2-yl)]-2,2’-iminodiethanol hydrochloride. Nebivolol is a racemate composed of d-Nebivolol and l-Nebivolol with the stereochemical designations of [SRRR]-nebivolol and [RSSS]-nebivolol, respectively. Nebivolol’s molecular formula is (C22H25F2NO4•HCl) with the following structural formula:
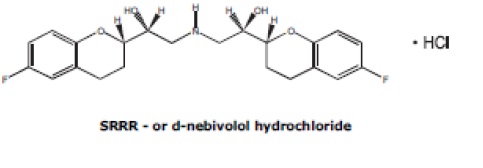
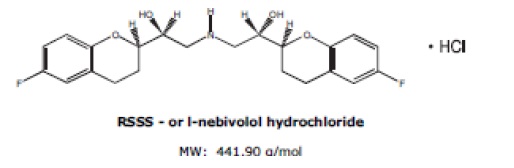
Nebivolol hydrochloride is a white to almost white powder that is soluble in
methanol, dimethylsulfoxide, and N,N-dimethylformamide, sparingly soluble in
ethanol, propylene glycol, and polyethylene glycol, and very slightly soluble
in hexane, dichloromethane, and methylbenzene.
NEBIVOLOL as tablets for oral administration contains nebivolol hydrochloride
equivalent to 2.5, 5, 10, and 20 mg of nebivolol base. In addition, nebivolol
tablets contains the following inactive ingredients: colloidal silicon
dioxide, croscarmellose sodium, D & C Red #27 Lake, FD & C Blue #2 Lake, FD &
C Yellow #6 Lake, hydroxypropyl methyl cellulose, lactose monohydrate,
magnesium stearate, microcrystalline cellulose, polysorbate 80, pregelatinized
starch.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
Nebivolol is a β-adrenergic receptor blocking agent. In extensive metabolizers (most of the population) and at doses less than or equal to 10 mg, nebivolol is preferentially β1 selective. In poor metabolizers and at higher doses, nebivolol inhibits both β1 - and β2 - adrenergic receptors. Nebivolol lacks intrinsic sympathomimetic and membrane stabilizing activity at therapeutically relevant concentrations. At clinically relevant doses, nebivolol tablets do not demonstrate α1-adrenergic receptor blockade activity. Various metabolites, including glucuronides, contribute to β-blocking activity.
12.1 Mechanism of Action
The mechanism of action of the antihypertensive response of nebivolol tablets has not been definitively established. Possible factors that may be involved include: (1) decreased heart rate, (2) decreased myocardial contractility, (3) diminution of tonic sympathetic outflow to the periphery from cerebral vasomotor centers, (4) suppression of renin activity and (5) vasodilation and decreased peripheral vascular resistance.
12.3 Pharmacokinetics
Nebivolol is metabolized by a number of routes, including glucuronidation and
hydroxylation by CYP2D6. The active isomer (d-nebivolol) has an effective
half-life of about 12 hours in CYP2D6 extensive metabolizers (most people),
and 19 hours in poor metabolizers and exposure to d-nebivolol is substantially
increased in poor metabolizers. This has less importance than usual, however,
because the metabolites, including the hydroxyl metabolite and glucuronides
(the predominant circulating metabolites), contribute to β-blocking activity.
Plasma levels of d–nebivolol increase in proportion to dose in EMs and PMs for
doses up to 20mg. Exposure to l-nebivolol is higher than to d-nebivolol but
l-nebivolol contributes little to the drug’s activity as d-nebivolol’s beta
receptor affinity is > 1000-fold higher than l-nebivolol. For the same dose,
PMs attain a 5-fold higher Cmax and 10-fold higher AUC of d-nebivolol than do
EMs. d-Nebivolol accumulates about 1.5-fold with repeated once-daily dosing in
EMs.
Absorption
Absorption of nebivolol tablets is similar to an oral solution. The absolute
bioavailability has not been determined.
Mean peak plasma nebivolol concentrations occur approximately 1.5 to 4 hours
post-dosing in EMs and PMs.
Food does not alter the pharmacokinetics of nebivolol. Under fed conditions,
nebivolol glucuronides are slightly reduced. Nebivolol tablets may be
administered without regard to meals.
Distribution
The in vitro human plasma protein binding of nebivolol is approximately 98%,
mostly to albumin, and is independent of nebivolol concentrations.
Metabolism
Nebivolol is predominantly metabolized via direct glucuronidation of parent
and to a lesser extent via N-dealkylation and oxidation via cytochrome P450
2D6. Its stereospecific metabolites contribute to the pharmacologic activity
[see Drug Interactions (7)].
Elimination
After a single oral administration of 14C-nebivolol, 38% of the dose was
recovered in urine and 44% in feces for EMs and 67% in urine and 13% in feces
for PMs. Essentially all nebivolol was excreted as multiple oxidative
metabolites or their corresponding glucuronide conjugates.
12.4 Pharmacokinetics in Special Populations
Hepatic Disease
d-Nebivolol peak plasma concentration increased 3-fold, exposure (AUC)
increased 10-fold, and the apparent clearance decreased by 86% in patients
with moderate hepatic impairment (Child-Pugh Class B). No formal studies have
been performed in patients with severe hepatic impairment and nebivolol should
be contraindicated for these patients [see Dosage and Administration (2.1)].
Renal Disease
The apparent clearance of nebivolol was unchanged following a single 5 mg dose
of nebivolol tablets in patients with mild renal impairment (ClCr 50 to 80
mL/min, n=7), and it was reduced negligibly in patients with moderate (ClCr 30
to 50 mL/min, n=9), but clearance was reduced by 53% in patients with severe
renal impairment (ClCr <30 mL/min, n=5). No studies have been conducted in
patients on dialysis [see Dosage and Administration (2.1)].
12.5 Drug-Drug Interactions
Drugs that inhibit CYP2D6 can be expected to increase plasma levels of
nebivolol. When nebivolol tablets are co-administered with an inhibitor or an
inducer of this enzyme, monitor patients closely and adjust the nebivolol dose
according to blood pressure response. In vitro studies have demonstrated that
at therapeutically relevant concentrations, d- and l-nebivolol do not inhibit
any cytochrome P450 pathways.
Digoxin: Concomitant administration of nebivolol tablets (10 mg once daily)
and digoxin (0.25 mg once daily) for 10 days in 14 healthy adult individuals
resulted in no significant changes in the pharmacokinetics of digoxin or
nebivolol [see Drug Interactions (7)].
Warfarin: Administration of nebivolol tablets (10 mg once daily for 10 days)
led to no significant changes in the pharmacokinetics of nebivolol or R- or
S-warfarin following a single 10 mg dose of warfarin. Similarly, nebivolol has
no significant effects on the anticoagulant activity of warfarin, as assessed
by Prothrombin time and INR profiles from 0 to 144 hours after a single 10 mg
warfarin dose in 12 healthy adult volunteers.
Diuretics: No pharmacokinetic interactions were observed in healthy adults
between nebivolol (10 mg daily for 10 days) and furosemide (40 mg single
dose), hydrochlorothiazide (25 mg once daily for 10 days), or spironolactone
(25 mg once daily for 10 days).
Ramipril: Concomitant administration of nebivolol tablets (10 mg once daily)
and ramipril (5 mg once daily) for 10 days in 15 healthy adult volunteers
produced no pharmacokinetic interactions.
Losartan: Concomitant administration of nebivolol tablets (10 mg single dose)
and losartan (50 mg single dose) in 20 healthy adult volunteers did not result
in pharmacokinetic interactions.
Fluoxetine: Fluoxetine, a CYP2D6 inhibitor, administered at 20 mg per day for
21 days prior to a single 10 mg dose of nebivolol to 10 healthy adults, led to
an 8-fold increase in the AUC and 3-fold increase in Cmax for d-nebivolol [see Drug Interactions (7)].
Histamine-2 Receptor Antagonists: The pharmacokinetics of nebivolol (5 mg
single dose) were not affected by the co-administration of ranitidine (150 mg
twice daily). Cimetidine (400 mg twice daily) causes a 23% increase in the
plasma levels of d-nebivolol.
Charcoal: The pharmacokinetics of nebivolol (10 mg single dose) were not
affected by repeated co-administration (4, 8, 12, 16, 22, 28, 36, and 48 hours
after nebivolol administration) of activated charcoal (Actidose®-Aqua).
Sildenafil: The co-administration of nebivolol and sildenafil decreased AUC
and Cmax of sildenafil by 21 and 23% respectively. The effect on the Cmax and
AUC for d-nebivolol was also small (< 20%). The effect on vital signs (e.g.,
pulse and blood pressure) was approximately the sum of the effects of
sildenafil and nebivolol.
Other Concomitant Medications: Utilizing population pharmacokinetic analyses,
derived from hypertensive patients, the following drugs were observed not to
have an effect on the pharmacokinetics of nebivolol: acetaminophen,
acetylsalicylic acid, atorvastatin, esomeprazole, ibuprofen, levothyroxine
sodium, metformin, sildenafil, simvastatin, or tocopherol.
Protein Binding: No meaningful changes in the extent of in vitro binding of
nebivolol to human plasma proteins were noted in the presence of high
concentrations of diazepam, digoxin, diphenylhydantoin, enalapril,
hydrochlorothiazide, imipramine, indomethacin, propranolol, sulfamethazine,
tolbutamide, or warfarin. Additionally, nebivolol did not significantly alter
the protein binding of the following drugs: diazepam, digoxin,
diphenylhydantoin, hydrochlorothiazide, imipramine, or warfarin at their
therapeutic concentrations.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a two-year study of nebivolol in mice, a statistically significant increase
in the incidence of testicular Leydig cell hyperplasia and adenomas was
observed at 40 mg/kg/day (5 times the maximally recommended human dose of 40
mg on a mg/m2 basis). Similar findings were not reported in mice administered
doses equal to approximately 0.3 or 1.2 times the maximum recommended human
dose. No evidence of a tumorigenic effect was observed in a 24-month study in
Wistar rats receiving doses of nebivolol 2.5, 10 and 40 mg/kg/day (equivalent
to 0.6, 2.4, and 10 times the maximally recommended human dose). Co-
administration of dihydrotestosterone reduced blood LH levels and prevented
the Leydig cell hyperplasia, consistent with an indirect LH-mediated effect of
nebivolol in mice and not thought to be clinically relevant in man.
A randomized, double-blind, placebo- and active-controlled, parallel-group
study in healthy male volunteers was conducted to determine the effects of
nebivolol on adrenal function, luteinizing hormone, and testosterone levels.
This study demonstrated that 6 weeks of daily dosing with 10 mg of nebivolol
had no significant effect on ACTH-stimulated mean serum cortisol AUC0-120 min,
serum LH, or serum total testosterone.
Effects on spermatogenesis were seen in male rats and mice at ≥ 40 mg/kg/day
(10 and 5 times the MRHD, respectively). For rats the effects on
spermatogenesis were not reversed and may have worsened during a four week
recovery period. The effects of nebivolol on sperm in mice, however, were
partially reversible.
Mutagenesis: Nebivolol was not genotoxic when tested in a battery of assays
(Ames, in vitro mouse lymphoma TK+/-, in vitro human peripheral lymphocyte
chromosome aberration, in vivo Drosophila melanogaster sex-linked recessive
lethal, and in vivo mouse bone marrow micronucleus tests).
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Hypertension
The antihypertensive effectiveness of nebivolol tablets as monotherapy has
been demonstrated in three randomized, double-blind, multi-center, placebo-
controlled trials at doses ranging from 1.25 to 40 mg for 12 weeks (Studies 1,
2, and 3). A fourth placebo-controlled trial demonstrated additional
antihypertensive effects of nebivolol tablets at doses ranging from 5 to 20 mg
when administered concomitantly with up to two other antihypertensive agents
(ACE inhibitors, angiotensin II receptor antagonists, and thiazide diuretics)
in patients with inadequate blood pressure control.
The three monotherapy trials included a total of 2016 patients (1811 nebivolol
tablets, 205 placebo) with mild to moderate hypertension who had baseline
diastolic blood pressures (DBP) of 95 to 109 mmHg. Patients received either
nebivolol tablets or placebo once daily for twelve weeks. Two of these
monotherapy trials (Studies 1 and 2) studied 1716 patients in the general
hypertensive population with a mean age of 54 years, 55% males, 26% non-
Caucasians, 7% diabetics and 6% genotyped as PMs. The third monotherapy trial
(Study 3) studied 300 Black patients with a mean age of 51 years, 45% males,
14% diabetics, and 3% as PMs.
Placebo-subtracted blood pressure reductions by dose for each study are
presented inTable 2. Most studies showed increasing response to doses
above 5 mg.
Table 2. Placebo-Subtracted Least-Square Mean Reductions in Trough Sitting Systolic/Diastolic Blood Pressure (SiSBP/SiDBP mmHg) by Dose in Studies with Once Daily Nebivolol Tablets
|
Nebivolol dose (mg) | ||||||
|
1.25 |
2.5 |
5.0 |
10 |
20 |
30-40 | |
|
Study 1 |
-6.6*/-5.1* |
-8.5*/-5.6* |
-8.1*/-5.5* |
-9.2*/-6.3* |
-8.7*/-6.9* |
-11.7*/-8.3* |
|
Study 2 |
-3.8/-3.2* |
-3.1/-3.9* |
-6.3*/-4.5* | |||
|
Study 3¶ |
-1.5/-2.9 |
-2.6/-4.9* |
-6.0*/-6.1* |
-7.2*/-6.1* |
-6.8*/-5.5* | |
|
Study 4^ |
-5.7*/-3.3* |
-3.7*/-3.5* |
-6.2*/-4.6* |
- p<0.05 based on pair-wise comparison vs. placebo
¶ Study enrolled only African Americans.
^ Study on top of one or two other antihypertensive medications.****
Study 4 enrolled 669 patients with a mean age of 54 years, 55% males, 54%
Caucasians, 29% Blacks, 15% Hispanics, 1% Asians, 14% diabetics, and 5% PMs.
Nebivolol tablets, 5 mg to 20 mg, administered once daily concomitantly with
stable doses of up to two other antihypertensive agents (ACE inhibitors,
angiotensin II receptor antagonists, and thiazide diuretics) resulted in
significant additional antihypertensive effects over placebo compared to
baseline blood pressure.
Effectiveness was similar in subgroups analyzed by age and sex. Effectiveness
was established in Blacks, but as monotherapy the magnitude of effect was
somewhat less than in Caucasians.
The blood pressure lowering effect of nebivolol tablets was seen within two
weeks of treatment and was maintained over the 24-hour dosing interval.
There are no trials of nebivolol tablets demonstrating reductions in
cardiovascular risk in patients with hypertension, but at least one
pharmacologically similar drug has demonstrated such benefits.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Nebivolol tablets are available as tablets for oral administration containing nebivolol hydrochloride equivalent to 2.5, 5, 10, and 20 mg of nebivolol.
Nebivolol Tablets, 2.5 mg are light blue colored mottled, triangular shape,
biconvex uncoated tablets debossed with “I 7” on one side and plain on the
other side.
Bottle of 30s NDC 33342-457-07
Bottle of 90s NDC 33342-457-10
Unit- Dose Carton of 12 x 10s NDC 33342-457-25
Nebivolol Tablets, 5 mg are beige colored mottled, triangular shape, biconvex
uncoated tablets debossed with “I 8” on one side and plain on the other side
Bottle of 30s NDC 33342-458-07
Bottle of 90s NDC 33342-458-10
Unit- Dose Carton of 12 x 10s NDC 33342-458-25
Nebivolol Tablets, 10 mg are pinkish purple colored mottled, triangular shape,
biconvex uncoated tablets debossed with “I 9” on one side and plain on the
other side.
Bottle of 30s NDC 33342-459-07
Bottle of 90s NDC 33342-459-10
Unit- Dose Carton of 12 x 10s NDC 33342-459-25
Nebivolol Tablets, 20 mg are light blue colored mottled, triangular shape,
biconvex uncoated tablets debossed with “I 10” on one side and plain on the
other side
Bottle of 30s NDC 33342-460-07
Bottle of 90s NDC 33342-460-10
Unit- Dose Carton of 12 x 10s NDC 33342-460-25
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59°
to 86°F) [see USP Controlled Room Temperature].
Dispense in a tight, light-resistant container as defined in the USP using a
child-resistant closure.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information).
•** Patient Advice**
Advise patients to take nebivolol tablets regularly and continuously, as
directed. Nebivolol tablets can be taken with or without food. If a dose is
missed, take the next scheduled dose only (without doubling it). Do not
interrupt or discontinue nebivolol tablets without consulting the physician.
Patients should know how they react to this medicine before they operate
automobiles, use machinery, or engage in other tasks requiring alertness.
Advise patients to consult a physician if any difficulty in breathing occurs,
or if they develop signs or symptoms of worsening congestive heart failure
such as weight gain or increasing shortness of breath, or excessive
bradycardia.
Caution patients subject to spontaneous hypoglycemia, or diabetic patients
receiving insulin or oral hypoglycemic agents, that β-blockers may mask some
of the manifestations of hypoglycemia, particularly tachycardia.
Inform patients or caregivers that there is a risk of hypoglycemia when
nebivolol tablets are given to patients who are fasting or who are vomiting.
Instruct patients or caregivers how to monitor for signs of hypoglycemia [see Warnings and Precautions (5.5)].
Manufactured for :
Macleods Pharma USA, Inc.
Princeton, NJ 08540
Manufactured by:
Macleods Pharmaceuticals Ltd.
Baddi, Himachal Pradesh-174101 INDIA
All the trademarks mentioned herewith are property of their respective owners.
SPL PATIENT PACKAGE INSERT SECTION
SPL PATIENT PACKAGE INSERT SECTION
PATIENT INFORMATION
** NEBIVOLOL (ne biv′ oh lol) TABLETS**
Read the Patient Information that comes with nebivolol tablets before you start taking it and each time you get a refill. There may be new information. This information does not take the place of talking with your doctor about your medical condition or your treatment. If you have any questions about nebivolol tablets, ask your doctor or pharmacist.
WHAT ARE NEBIVOLOL TABLETS?
Nebivolol tablets are a kind of prescription medicine called a “beta-blocker”.
Nebivolol tablets treats:
• High blood pressure (hypertension)
Nebivolol tablets can lower blood pressure when used by itself and with other medicines.
Nebivolol tablets are not approved for children less than 18 years of age.
WHO SHOULD NOT TAKE NEBIVOLOL TABLETS?
Do not take nebivolol tablets if you:
• Have heart failure and are in the ICU or need medicines to keep up your
blood circulation
• Have a slow heartbeat or your heart skips beats (irregular heartbeat)
• Have severe liver damage
• Are allergic to any ingredient in nebivolol tablets. The active ingredient
is nebivolol. See the end of this leaflet for a list of ingredients.
WHAT SHOULD I TELL MY DOCTOR BEFORE TAKING NEBIVOLOL TABLETS?
Tell your doctor about all of your medical problems, including if you:
• Have asthma or other lung problems (such as bronchitis or emphysema)
• Have problems with blood flow in your feet and legs (peripheral vascular
disease) nebivolol tablets can make symptoms of blood flow problems worse.
• Have diabetes and take medicine to control blood sugar
• Have thyroid problems
• Have liver or kidney problems
• Had allergic reactions to medications or have allergies
• Have a condition called pheochromocytoma
• Are pregnant or trying to become pregnant. It is not known if nebivolol
tablets are safe for your unborn baby. Talk with your doctor about the best
way to treat high blood pressure while you are pregnant.
• Are breastfeeding. It is not known if nebivolol passes into your breast
milk. You should not breastfeed while using nebivolol tablets.
• Are scheduled for surgery and will be given anesthetic agents
Tell your doctor about all the medicines you take. Include prescription
and non-prescription medicines, vitamins, and herbal products. Nebivolol
tablets and certain other medicines can affect each other and cause serious
side effects.
Keep a list of all the medicines you take. Show this list to your doctor and
pharmacist before you start a new medicine.
HOW SHOULD I TAKE NEBIVOLOL TABLETS?
•Do not suddenly stop taking nebivolol tablets. You could have chest pain
or a heart attack. If your doctor decides to stop nebivolol tablets, your
doctor may slowly lower your dose over time before stopping it completely.
•Take nebivolol tablets every day exactly as your doctor tells you. Your
doctor will tell you how much nebivolol tablets to take and how often. Your
doctor may start with a low dose and raise it over time.
• Do not stop taking nebivolol tablets or change your dose without talking
with your doctor.
• Take nebivolol tablets with or without food.
• If you miss a dose, take your dose as soon as you remember, unless it is
close to the time to take your next dose. Do not take 2 doses at the same
time. Take your next dose at the usual time.
• If you take too much nebivolol tablets, call your doctor or poison control
center right away.
WHAT ARE POSSIBLE SIDE EFFECTS OF NEBIVOLOL TABLETS?
• Low blood pressure and feeling dizzy. If you feel dizzy, sit or lie down and
tell your doctor right away.
• Tiredness
• Slow heartbeat
• Headache
• Leg swelling due to fluid retention (edema). Tell your doctor if you gain
weight or have trouble breathing while taking nebivolol tablets.
• If you are diabetic or take medicine for high blood sugar or if you have a
tendency to have low blood sugar, Nebivolol tablets can mask/cover some of the
signs and symptoms that would tell you that your blood sugar may be low, like
heart palpitations or rapid heart beating. Ask your doctor for other signs
that may alert you when having low blood sugar.
Tell your doctor if you have any side effects that bother you or don’t go
away.
HOW SHOULD I STORE NEBIVOLOL TABLETS?
• Store nebivolol tablets between 68° to 77°F (20° - 25°C).
• Safely throw away nebivolol tablets that are out of date or no longer
needed.
• Keep nebivolol tablets and all medicines out of the reach of children.
GENERAL INFORMATION ABOUT NEBIVOLOL TABLETS
Doctors sometimes prescribe medicines for conditions not included in the
patient information leaflets.
• Only use nebivolol tablets for the medical problem it was prescribed for.
• Do not give nebivolol tablets to other people, even if they have the same
symptoms. It may harm them.
This leaflet summarizes the most important information about nebivolol
tablets. For more information:
• Talk with your doctor.
• Ask your doctor or pharmacist for information about nebivolol tablets that
is written for healthcare professionals.
• For more information, call 1-888-943-3210 or 1-855-926-3384.
WHAT IS IN NEBIVOLOL TABLETS?
Active Ingredient: Nebivolol
**Inactive Ingredients:**colloidal silicon dioxide, croscarmellose sodium, D
& C Red #27 Lake, FD & C Blue #2 Lake, FD & C Yellow #6 Lake, hydroxypropyl
methyl cellulose, lactose monohydrate, magnesium stearate, microcrystalline
cellulose, polysorbate 80, pregelatinized starch.
WHAT IS HIGH BLOOD PRESSURE (HYPERTENSION)?
Blood pressure is the force in your blood vessels when your heart beats and
when your heart rests. You have high blood pressure when the force is too
great.
High blood pressure makes the heart work harder to pump blood through the body
and causes damage to the blood vessels. Nebivolol tablets can help your blood
vessels relax so your blood pressure is lower. Medicines that lower your blood
pressure lower your chance of having a stroke or heart attack.
Manufactured for :
Macleods Pharma USA, Inc.
Princeton, NJ 08540
Manufactured by:
Macleods Pharmaceuticals Ltd.
Baddi, Himachal Pradesh-174101 INDIA
Revised. 01/2025
RECENT MAJOR CHANGES SECTION
RECENT MAJOR CHANGES
Warnings and Precautions, Hypoglycemia (5.5) 6/2023
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Hypertension
The dose of nebivolol tablets must be individualized to the needs of the
patient. For most patients, the recommended starting dose is 5 mg once daily,
with or without food, as monotherapy or in combination with other agents. For
patients requiring further reduction in blood pressure, the dose can be
increased at 2-week intervals up to 40 mg. A more frequent dosing regimen is
unlikely to be beneficial.
Renal Impairment
In patients with severe renal impairment (ClCr less than 30 mL/min) the
recommended initial dose is 2.5 mg once daily; titrate up slowly if needed.
Nebivolol tablets have not been studied in patients receiving dialysis [see Clinical Pharmacology (12.4)].
Hepatic Impairment
In patients with moderate hepatic impairment, the recommended initial dose is
2.5 mg once daily; titrate up slowly if needed. Nebivolol tablets have not
been studied in patients with severe hepatic impairment and therefore it is
not recommended in that population [see Clinical Pharmacology (12.4)].
2.2 Subpopulations
Geriatric Patients
It is not necessary to adjust the dose in the elderly [see use in Specific Populations (8.5)].
CYP2D6 Polymorphism
No dose adjustments are necessary for patients who are CYP2D6 poor
metabolizers. The clinical effect and safety profile observed in poor
metabolizers were similar to those of extensive metabolizers [see Clinical Pharmacology (12.3)].
Can be taken with and without food. Individualize to the needs of the patient
and monitor during up-titration. (2)
• Hypertension: Most patients start at 5 mg once daily. Dose can be increased
at 2-week intervals up to 40 mg. (2.1)
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
Nebivolol Tablets are available as tablets for oral administration containing nebivolol hydrochloride equivalent to 2.5, 5, 10, and 20 mg of nebivolol.
Nebivolol Tablets, 2.5 mg are light blue colored mottled, triangular shape, biconvex uncoated tablets debossed with “I 7” on one side and plain on the other side.
Nebivolol Tablets, 5 mg are beige colored mottled, triangular shape, biconvex uncoated tablets debossed with “I 8” on one side and plain on the other side
Nebivolol Tablets, 10 mg are pinkish purple colored mottled, triangular shape, biconvex uncoated tablets debossed with “I 9” on one side and plain on the other side.
Nebivolol Tablets, 20 mg are light blue colored mottled, triangular shape, biconvex uncoated tablets debossed with “I 10” on one side and plain on the other side
Tablets: 2.5, 5, 10, 20 mg (3)
