tolvaptan
These highlights do not include all the information needed to use TOLVAPTAN TABLETS safely and effectively. See full prescribing information for TOLVAPTAN TABLETS. TOLVAPTAN tablets, for oral use Initial U.S. Approval: 2009
d9fd11d0-4269-12fb-95cf-b3c11d75e479
HUMAN PRESCRIPTION DRUG LABEL
Sep 22, 2025
Apotex Corp.
DUNS: 845263701
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
tolvaptan
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
tolvaptan
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL — 30 mg BLISTER CARTON LABEL
Representative sample of bottle carton (see HOW SUPPLIED section for complete listing):
NDC 60505-4318-0
Tolvaptan Tablets
30 mg
APOTEX CORP
Rx Only
SPL MEDGUIDE SECTION
MEDICATION GUIDE
********Tolvaptan (tol vap'tan)
Tablets
Read the Medication Guide that comes with Tolvaptan Tablets before you take it and each time you get a new prescription. There may be new information. This Medication Guide does not take the place of talking to your healthcare provider about your medical condition or your treatment. Share this important information with members of your household.
What is the most important information I should know abouttolvaptan tablets****?
****Tolvaptan tablets****** may make the salt (sodium) level in your blood rise too fast.**This can increase your risk of a serious condition called osmotic demyelination syndrome (ODS). ODS can lead to coma or death. ODS can also cause new symptoms such as:
- trouble speaking
- swallowing trouble or feeling like food or liquid gets stuck while swallowing
- drowsiness
- confusion
- mood changes
- trouble controlling body movement (involuntary movement) and weakness in muscles of the arms and legs
- seizures
You or a family member should tell your healthcare provider right away if you have any of these symptoms even if they begin later in treatment. Also tell your healthcare provider about any other new symptoms while taking tolvaptan tablets.
You may be more at risk for ODS if you have:
- liver disease
- not eaten enough for a long period of time (malnourished)
- very low sodium level in your blood
- been drinking large amounts of alcohol for a long period of time (chronic alcoholism)
To lessen your risk of ODS while taking tolvaptan tablets:
Treatment withtolvaptan tablets*** should be started and re-started only in a hospital, where the sodium levels in your blood can be checked closely.**
- Do not take tolvaptan tablets if you cannot tell if you are thirsty.
- To prevent losing too much body water (dehydration), have water available to drink at all times while taking tolvaptan tablets. Unless your healthcare provider tells you otherwise, drink when you are thirsty.
- If your healthcare provider tells you to keep taking tolvaptan tablets after you leave a hospital, it is important that you do not stop and re-start tolvaptan tablets on your own. You may need to go back to a hospital to re-start tolvaptan tablets. Talk to your healthcare provider right away if you stop taking tolvaptan tablets for any reason.
- It is important to stay under the care of your healthcare provider while taking tolvaptan tablets and follow their instructions.
****Tolvaptan tablets****** may cause liver problems, including life-threatening liver failure.**Tolvaptan tablets should not be taken for more than 30 days. Tell your doctor right away if you develop or have worsening of any of these signs and symptoms of liver problems:
- Loss of appetite, nausea, vomiting
- Fever, feeling unwell, unusual tiredness
- Itching
- Yellowing of the skin or the whites of the eyes (jaundice)
- Unusual darkening of the urine
- Right upper stomach area pain or discomfort
** If you have autosomal dominant polycystic kidney disease (ADPKD), do not use Tolvaptan tablets because you should receive the medicine (tolvaptan) through a program that ensures laboratory monitoring of your liver.**
What aretolvaptan tablets****?
Tolvaptan tablets are a prescription medicine used to help increase low sodium levels in the blood, in adults with conditions such as heart failure, and certain hormone imbalances. Tolvaptan tablets helps raise salt levels in your blood by removing extra body water as urine.
It is not known if tolvaptan tablets are safe or works in children.
Who should not taketolvaptan tablets****?
Do not take tolvaptan tablets if:
- you are allergic to tolvaptan or any of the ingredients in tolvaptan tablets. See the end of this Medication Guide for a complete list of ingredients in tolvaptan tablets.
- the sodium level in your blood must be increased right away.
- you cannot replace fluids by drinking or you cannot feel if you are thirsty.
- you are dizzy, faint, or your kidneys are not working normally because you have lost too much body fluid.
- you take certain medicines. These medicines could cause you to have too much tolvaptan tablets in your blood:
- the antibiotic medicines, clarithromycin (Biaxin, Biaxin XL) or telithromycin (Ketek)
- the antifungal medicines, ketoconazole (Nizoral) or itraconazole (Sporonox)
- the anti-HIV medicines, ritonavir (Kaletra, Norvir), indinavir (Crixivan), nelfinavir (Viracept), and saquinavir (Invirase)
- the antidepressant medicine, nefazodone hydrochloride
- your body is not able to make urine. Tolvaptan tablets will not help your condition.
What should I tell my healthcare provider before takingtolvaptan tablets****?
Tell your healthcare provider about all your medical conditions, including if you:
- have kidney problems and your body cannot make urine.
- have liver problems
- cannot feel if you are thirsty. See "What is the most important information I should know about tolvaptan tablets?"
- had an allergic reaction in the past to tolvaptan or any of the other ingredients of this medicine. See the end of this Medication Guide for a list of the ingredients in tolvaptan tablets.
- are pregnant or plan to become pregnant. It is not known if tolvaptan tablets will harm your unborn baby.
- are breast-feeding. It is not known if tolvaptan tablets passes into your breast milk. You and your healthcare provider should decide if you will take tolvaptan tablets or breast-feed. You should not do both.
- are taking desmopressin (dDAVP).
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
Using tolvaptan tablets with certain medicines could cause you to have too much tolvaptan tablets in your blood. See "Who should not take tolvaptan tablets?"
Tolvaptan tablets may affect the way other medicines work and other medicines may affect how tolvaptan tablets works.
Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I take tolvaptan tablets?
- See "What is the most important information I should know about tolvaptan tablets?"
- Take tolvaptan tablets exactly as prescribed by your healthcare provider.
- Take tolvaptan tablets one time each day.
- You can take tolvaptan tablets with or without food.
- Do not drink grapefruit juice during treatment with tolvaptan tablets. This could cause you to have too much tolvaptan tablets in your blood.
- Certain medicines or illnesses may keep you from drinking fluids or may cause you to lose too much body fluid, such as vomiting or diarrhea. If you have these problems, call your healthcare provider right away.
- Do not miss or skip doses of tolvaptan tablets. If you miss a dose, take it as soon as you remember. If it is near the time of the next dose, skip the missed dose. Just take the next dose at your regular time. Do not take 2 doses at the same time. *If you take too much tolvaptan tablets, call your healthcare provider right away. If you take an overdose of tolvaptan tablets, you may need to go to a hospital.
- If your healthcare provider tells you to stop taking tolvaptan tablets, follow their instructions about limiting the amount of fluid you should drink.
What are the possible side effects of tolvaptan tablets?
Tolvaptan tablets can cause serious side effects including:
*See "What is the most important information I should know about tolvaptan tablets?" *Loss of too much body fluid (dehydration). Tell your healthcare provider if you: * have vomiting or diarrhea and cannot drink normally. * feel dizzy or faint. These may be symptoms that you have lost too much body fluid.
Call your healthcare provider right away, if you have any of these symptoms.
The most common side effects of tolvaptan tablets are:
- thirst
- dry mouth
- weakness
- constipation
- making large amounts of urine and urinating often
- increased blood sugar levels
These are not all the possible side effects of tolvaptan tablets. Talk to your healthcare provider about any side effect that bothers you or that does not go away while taking tolvaptan tablets.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store tolvaptan tablets?
Store tolvaptan tablets between 59°F to 86°F (15°C to 30°C).
Keep tolvaptan tablets and all medicines out of the reach of children.
General Information about tolvaptan tablets
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use tolvaptan tablets for a condition for which it was not prescribed. Do not give tolvaptan tablets to other people, even if they have the same symptoms you have. It may harm them.
This Medication Guide summarizes the most important information about tolvaptan tablets. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about tolvaptan tablets that is written for healthcare professionals. For more information about tolvaptan tablets, call 1-800-706-5575 or go to www.apotex.com.
What are the ingredients in tolvaptan tablets?
Active ingredient: tolvaptan.
Inactive ingredients: anhydrous lactose, copovidone, colloidal silicon dioxide, croscarmellose sodium, indigotine al lake 12-14% and magnesium stearate.
Rx Only
APOTEX INC.
Tolvaptan Tablets 15 mg and 30 mg
|
Manufactured by: |
Manufactured for: |
|
Apotex Inc. |
Apotex Corp. |
Revised: November 2022
Rev. 4
DESCRIPTION SECTION
11 DESCRIPTION
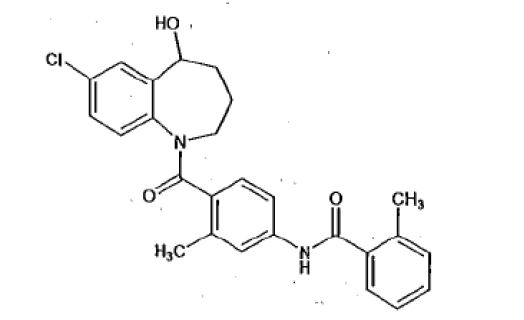
Tolvaptan tablets contain tolvaptan, a selective vasopressin V2-receptor antagonist in tablets for oral use available in 15 mg or 30 mg strengths. Tolvaptan is 7-Chloro-5-hydroxy-1-[2-methyl-4-(2-methylbenzoylamino)benzoyl]-2,3,4,5-tetrahydro-1H-1-benzazepine. The empirical formula is C26H25ClN2O3. Molecular weight is 448.95 g/mol. The chemical structure is:
Tolvaptan tablets for oral use contain 15 mg or 30 mg of tolvaptan. Inactive ingredients include anhydrous lactose, colloidal silicon dioxide, copovidone, croscarmellose sodium, indigotine al lake 12-14% and magnesium stearate.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Hyponatremia
In two double-blind, placebo-controlled, multi-center studies (SALT-1 and SALT-2), a total of 424 patients with euvolemic or hypervolemic hyponatremia (serum sodium <135 mEq/L) resulting from a variety of underlying causes (heart failure, liver cirrhosis, syndrome of inappropriate antidiuretic hormone [SIADH] and others) were treated for 30 days with tolvaptan or placebo, then followed for an additional 7 days after withdrawal. Symptomatic patients, patients likely to require saline therapy during the course of therapy, patients with acute and transient hyponatremia associated with head trauma or postoperative state and patients with hyponatremia due to primary polydipsia, uncontrolled adrenal insufficiency or uncontrolled hypothyroidism were excluded. Patients were randomized to receive either placebo (N = 220) or tolvaptan (N = 223) at an initial oral dose of 15 mg once daily. The mean serum sodium concentration at study entry was 129 mEq/L. Fluid restriction was to be avoided if possible during the first 24 hours of therapy to avoid overly rapid correction of serum sodium, and during the first 24 hours of therapy 87% of patients had no fluid restriction. Thereafter, patients could resume or initiate fluid restriction (defined as daily fluid intake of ≤1.0 liter/day) as clinically indicated.
The dose of tolvaptan could be increased at 24 hour intervals to 30 mg once daily, then to 60 mg once daily, until either the maximum dose of 60 mg or normonatremia (serum sodium >135 mEq/L) was reached. Serum sodium concentrations were determined at 8 hours after study drug initiation and daily up to 72 hours, within which time titration was typically completed. Treatment was maintained for 30 days with additional serum sodium assessments on Days 11, 18, 25 and 30. On the day of study discontinuation, all patients resumed previous therapies for hyponatremia and were reevaluated 7 days later. The primary endpoint for these studies was the average daily AUC for change in serum sodium from baseline to Day 4 and baseline to Day 30 in patients with a serum sodium less than 135 mEq/L. Compared to placebo, tolvaptan caused a statistically greater increase in serum sodium (p <0.0001) during both periods in both studies (see Table 2). For patients with a serum sodium of <130 mEq/L or <125 mEq/L, the effects at Day 4 and Day 30 remained significant (see Table 2). This effect was also seen across all disease etiology subsets (e.g., CHF, cirrhosis, SIADH/other).
Table 2. Effects of Treatment with Tolvaptan 15 mg/day to 60 mg/day|
Tolvaptan |
Placebo |
Estimated Effect | |
|---|---|---|---|
| |||
|
Subjects with Serum Sodium <135 mEq/L (ITT population) | |||
|
Change in average daily serum [Na+] AUC baseline to Day 4 (mEq/L) |
4.0 (2.8) |
0.4 (2.4) |
3.7 (3.3 to 4.2) |
|
Change in average daily serum [Na+] AUC baseline to Day 30 (mEq/L) |
6.2 (4.0) |
1.8 (3.7) |
4.6 (3.9 to 5.2) |
|
Percent of Patients Needing Fluid Restriction* |
14% |
25% |
p =0.0017 |
|
Subgroup with Serum Sodium <130 mEq/L | |||
|
Change in average daily serum [Na+] AUC baseline to Day 4 (mEq/L) |
4.8 (3.0) |
0.7 (2.5) |
4.2 (3.5 to 5.0) |
|
Change in average daily serum [Na+] AUC baseline to Day 30 (mEq/L) |
7.9 (4.1) |
2.6 (4.2) |
5.5 (4.4 to 6.5) |
|
Percent of Patients Needing Fluid Restriction |
19% |
36% |
p <0.01 |
|
Subgroup with Serum Sodium <125 mEq/L | |||
|
Change in average daily serum [Na+] AUC baseline to Day 4 (mEq/L) |
5.7 (3.8) |
1.0 (1.8) |
5.3 (3.8 to 6.9) |
|
Change in average daily serum [Na+] AUC baseline to Day 30 (mEq/L) |
10.0 (4.8) |
4.1 (4.5) |
5.7 (3.1 to 8.3) |
|
Percent of Patients Needing Fluid Restriction |
35% |
50% |
p = 0.14 |
In patients with hyponatremia (defined as <135 mEq/L), serum sodium concentration increased to a significantly greater degree in tolvaptan-treated patients compared to placebo-treated patients as early as 8 hours after the first dose, and the change was maintained for 30 days. The percentage of patients requiring fluid restriction (defined as ≤1 L/day at any time during the treatment period) was also significantly less (p = 0.0017) in the tolvaptan-treated group (30/215, 14%) as compared with the placebo-treated group (51/206, 25%).
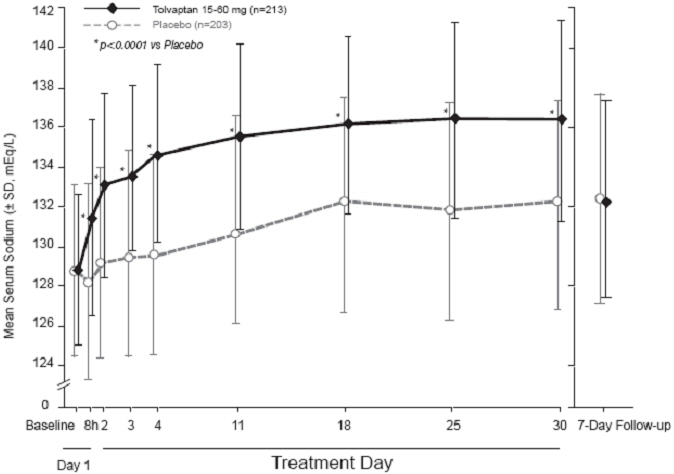
Figure 1 shows the change from baseline in serum sodium by visit in patients with serum sodium <135 mEq/L. Within 7 days of tolvaptan discontinuation, serum sodium concentrations in tolvaptan-treated patients declined to levels similar to those of placebo-treated patients.
|
Figure 1: Pooled SALT Studies: Analysis of Mean Serum Sodium (± SD, mEq/L) by Visit - Patients with Baseline Serum Sodium <135 mEq/L |
|
|
|
*p‑value <0.0001 for all visits during tolvaptan treatment compared to placebo |
|
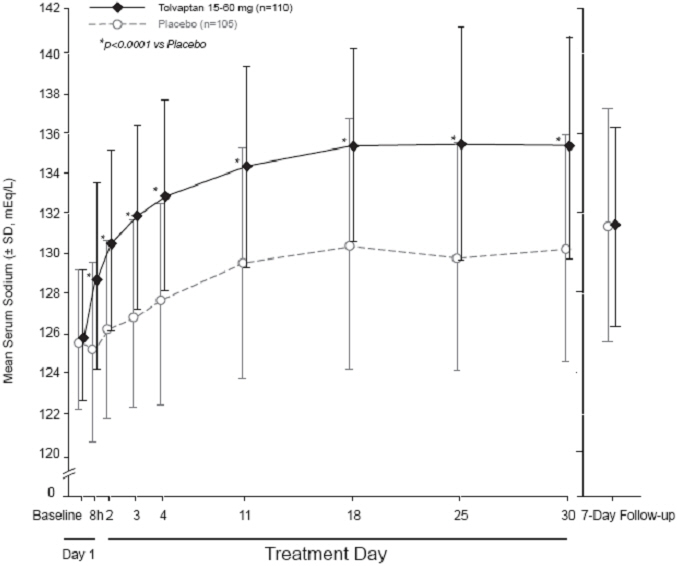
Figure 2: Pooled SALT Studies: Analysis of Mean Serum Sodium (± SD, mEq/L) by Visit - Patients with Baseline Serum Sodium <130 mEq/L |
|
|
|
*p‑value <0.0001 for all visits during tolvaptan treatment compared to placebo |
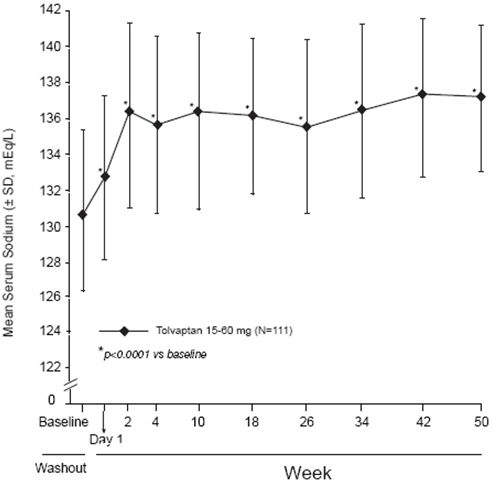
In the open-label study SALTWATER, 111 patients, 94 of them hyponatremic (serum sodium <135 mEq/L), previously on tolvaptan or placebo therapy, were given tolvaptan as a titrated regimen (15 to 60 mg once daily) after having returned to standard care for at least 7 days. By this time, their baseline mean serum sodium concentration had fallen to between their original baseline and post-placebo therapy level. Upon initiation of therapy, average serum sodium concentrations increased to approximately the same levels as observed for those previously treated with tolvaptan and were sustained for at least a year. Figure 3 shows results from 111 patients enrolled in the SALTWATER Study.
|
Figure 3: SALTWATER: Analysis of Mean Serum Sodium (± SD, mEq/L) by Visit |
|
|
|
*p‑value <0.0001 for all visits during tolvaptan treatment compared to baseline |
14.2 Heart Failure
In a phase 3 double-blind, placebo-controlled study (EVEREST), 4133 patients with worsening heart failure were randomized to tolvaptan or placebo as an adjunct to standard of care. Long-term tolvaptan treatment (mean duration of treatment of 0.75 years) had no demonstrated effect, either favorable or unfavorable, on all-cause mortality [HR (95% CI): 0.98 (0.9, 1.1)] or the combined endpoint of CV mortality or subsequent hospitalization for worsening HF [HR (95% CI): 1.0 (0.9, 1.1)].