Erythromycin Lactobionate
Erythromycin Lactobionate for Injection, USP For Intravenous Use Only Vials
171b8b28-9da6-4bc6-85ea-f27fde5d5f10
HUMAN PRESCRIPTION DRUG LABEL
Mar 9, 2023
Nexus Pharmaceuticals Inc.
DUNS: 620714787
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Erythromycin Lactobionate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (2)
Drug Labeling Information
DESCRIPTION SECTION
DESCRIPTION
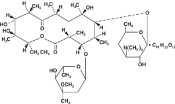
Erythromycin is produced by a strain of Streptomyces erythraeus and belongs to the macrolide group of antibiotics. It is basic and readily forms salts with acids.
Erythromycin lactobionate for injection, USP is a soluble salt of erythromycin suitable for intravenous administration. The lactobionic acid content is 244 mg per vial. Lactobionic acid and/or erythromycin are used to adjust the pH during the manufacture of the product. When reconstituted as directed, each mL contains 50 mg of erythromycin activity. The pH of the reconstituted solution is 6.5 -7.5.
Erythromycin lactobionate is chemically known as erythromycin mono (4-0-β-Dgalactopyranosyl-D-gluconate) (salt). The structural formula is: