Isoflurane
Isoflurane, USP Liquid for Inhalation Rx only
13c9c37a-0c3d-450a-a61e-d2796818b18f
HUMAN PRESCRIPTION DRUG LABEL
Sep 23, 2025
Piramal Critical Care Inc
DUNS: 805600439
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Isoflurane
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (1)
Drug Labeling Information
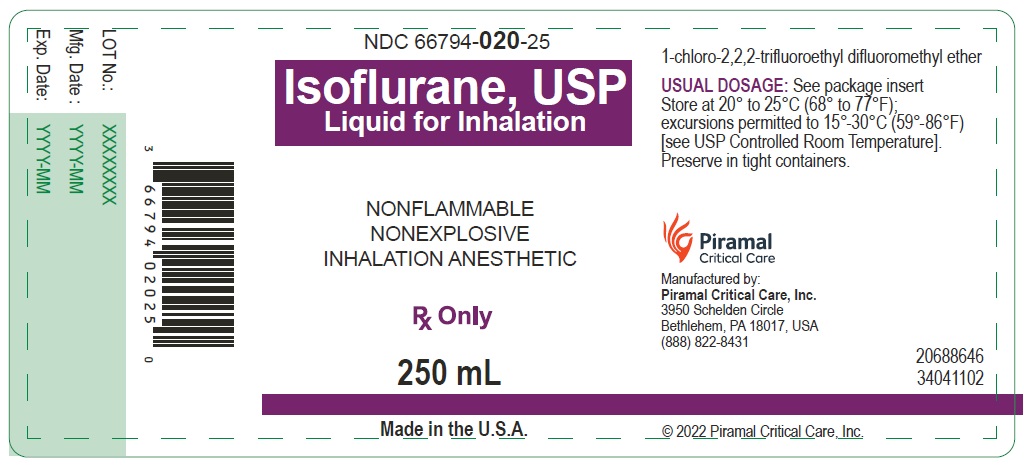
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPLE DISPLAY PANEL-250ML
Bottle Label
NDC 66794-020-25
Isoflurane, USP
Liquid for inhalation
250ml
Rx Only
DOSAGE & ADMINISTRATION SECTION
DOSAGE AND ADMINISTRATION
Isoflurane should be administered only by persons trained in the administration of general anesthesia.
Facilities for maintenance of a patent airway, artificial ventilation, oxygen enrichment, and circulatory resuscitation must be immediately available.
Isoflurane is administered by inhalation. Isoflurane should be delivered from a vaporizer specifically designed for use with isoflurane.
The minimum alveolar concentration (MAC) of isoflurane decreases with increasing patient age.
Dosage for induction and maintenance must be individualized and titrated to the desired effect according to the patient’s age and clinical status.
**Premedication:**Premedication should be selected according to the need of the individual patient, taking into account that secretions are weakly stimulated by isoflurane, and the heart rate tends to be increased.
**Induction:**Induction with isoflurane in oxygen or in combination with oxygen-nitrous oxide mixtures may produce coughing, breath holding, laryngospasm and bronchospasm, which increases with the concentration of isoflurane. These difficulties may be avoided by the use of a hypnotic dose of an ultra-short-acting barbiturate. Inspired concentrations of 1.5 to 3.0% isoflurane usually produce surgical anesthesia in 7 to 10 minutes.
Maintenance:
Isoflurane MAC values according to age are shown below:
|
Age |
Average MAC Value In 100% Oxygen |
Average MAC Value In 30% Oxygen and 70% N2O |
|
Preterm neonates ˂32 weeks gestational age |
1.28% | |
|
Preterm neonates 32-37 weeks gestational age |
1.41% | |
|
0-1 month |
1.60% | |
|
1-6 months |
1.87% | |
|
6-12 months |
1.80% | |
|
1-5 years |
1.60% | |
|
6-10 years |
1.45% | |
|
11-18 years |
1.38% | |
|
19-30 years |
1.28% |
0.56% |
|
31-55 years |
1.15% |
0.50% |
|
55-83 years |
1.05% |
0.37% |
Dosage for induction and maintenance must be individualized and titrated to the desired effect according to the patient’s age and clinical status.
Surgical levels of anesthesia may be sustained with a 1.0 to 2.5% concentration when nitrous oxide is used concomitantly. An additional 0.5 to 1.0% may be required when isoflurane is given using oxygen alone. If added relaxation is required, supplemental doses of muscle relaxants may be used.
The level of blood pressure during maintenance is an inverse function of isoflurane concentration in the absence of other complicating problems. Excessive decreases may be due to depth of anesthesia and in such instances may be corrected by lightening anesthesia.
DESCRIPTION SECTION
DESCRIPTION
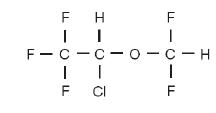
Isoflurane, USP, a nonflammable liquid administered by vaporizing, is a general inhalation anesthetic drug. It is 1-chloro-2,2,2-trifluoroethyl difluoromethyl ether, and its structural formula is:
Some physical constants are:
|
Molecular weight |
184.5 | |
|
Boiling point at 760 mm Hg |
48.5°C | |
|
Refractive index |
1.2990-1.3005 | |
|
Specific gravity 25°/25°C |
1.496 | |
|
Vapor pressure in mm Hg** |
20°C |
238 |
|
25°C |
295 | |
|
30°C |
367 | |
|
35°C |
450 |
**Equation for vapor pressure calculation:
log 10Pvap = A+ B where: A = 8.056
T B = -1664.58
T = °C + 273.16 (Kelvin)
|
Partition coefficients at 37**°**C | |
|
Water/gas |
0.61 |
|
Blood/gas |
1.43 |
|
Oil/gas |
90.8 |
|
Partition coefficients at 25**°**C - rubber and plastic | |
|
Conductive rubber/gas |
62.0 |
|
Butyl rubber/gas |
75.0 |
|
Polyvinyl chloride/gas |
110.0 |
|
Polyethylene/gas |
~2.0 |
|
Polyurethane/gas |
~1.4 |
|
Polyolefin/gas |
~1.1 |
|
Butyl acetate/gas |
~2.5 |
|
Purity by gas chromatography |
|
|
Lower limit of flammability in oxygen or nitrous oxide at 9 joules/sec. and 23°C |
None |
|
Lower limit of flammability in oxygen or nitrous oxide at 900 joules/sec. and 23°C |
Greater than useful concentration in anesthesia. |
Isoflurane is a clear, colorless, stable liquid containing no additives or chemical stabilizers. Isoflurane has a mildly pungent, musty, ethereal odor. Samples stored in indirect sunlight in clear, colorless glass for five years, as well as samples directly exposed for 30 hours to a 2 amp, 115 volt, 60 cycle long wave U.V. light were unchanged in composition as determined by gas chromatography. Isoflurane in one normal sodium methoxide-methanol solution, a strong base, for over six months consumed essentially no alkali, indicative of strong base stability. Isoflurane does not decompose in the presence of soda lime (at normal operating temperatures), and does not attack aluminum, tin, brass, iron or copper.