CloSYS
CloSYS Fluoride Toothpaste
5f87abb7-0e9d-6369-e053-2a91aa0a2411
HUMAN OTC DRUG LABEL
May 16, 2025
Rowpar Pharmaceuticals, Inc.
DUNS: 783704661
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Sodium Fluoride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
Drug Labeling Information
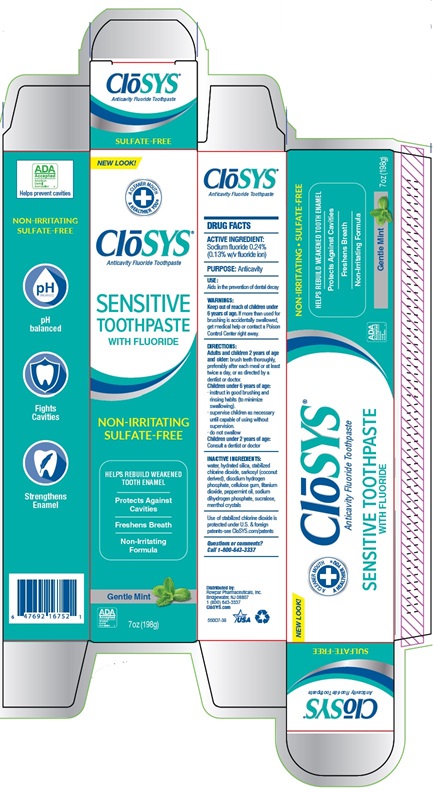
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal Display Panel
Closys®
Anticavity Fluoride Toothpaste
SULFATE-FREE
NEW LOOK!
A CLEANER MOUTH
A HEALTHIER YOU®
CLoSYS ®
Anticavity Fluoride Toothpaste
SENSITIVE
TOOTHPASTE
WITH FLUORIDE
NON-IRRITATING
SULFATE-FREE
HELPS REBUILD WEAKEND
TOOTH ENAMEL
Protects Against
Cavities
Freshens Breath
Non-Irritating
Formula
Gentle Mint
7oz (196g)
NON-IRRITATING
SULFATE-FREE
pH balanced
Fights
Cavities
Strengthens
Enamel
Closys®
Anticavity Fluoride Toothpaste
SENSITIVE TOOTHPASTE
WITH FLUORIDE
NON-IRRITATING SULFATE-FREE
HELPS REBUILD WEAKEND TOOTH ENAMEL
Protects Against Cavities
Freshens Breath
Non-Irritating Formula
Gentle Mint 7oz (196g)
Closys®
Anticavity Fluoride Toothpaste
SULFATE-FREE
NEW LOOK!
A CLEANER MOUTH
A HEALTHIER YOU®
CLoSYS ®
Anticavity Fluoride Toothpaste
SENSITIVE
TOOTHPASTE
WITH FLUORIDE
NON-IRRITATING
SULFATE-FREE
HELPS REBUILD WEAKEND
TOOTH ENAMEL
Protects Against
Cavities
Freshens Breath
Non-Irritating
Formula
Gentle Mint
3.4oz (96g)
NON-IRRITATING
SULFATE-FREE
pH balanced
Fights
Cavities
Strengthens
Enamel
Closys®
Anticavity Fluoride Toothpaste
SENSITIVE TOOTHPASTE
WITH FLUORIDE
NON-IRRITATING SULFATE-FREE
HELPS REBUILD WEAKEND TOOTH ENAMEL
Protects Against Cavities
Freshens Breath
Non-Irritating Formula
Gentle Mint 3.4oz (96g)

INDICATIONS & USAGE SECTION
USE:
Aids in the prevention of dental decay.
WARNINGS SECTION
WARNINGS:
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children under 6 years of age. If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
OTC - PURPOSE SECTION
PURPOSE:
Anticavity
OTC - ACTIVE INGREDIENT SECTION
ACTIVE INGREDIENT:
Sodium fluoride 0.24% (0.13% w/v fluoride ion)
DOSAGE & ADMINISTRATION SECTION
DIRECTIONS:
adults and children 2 years of age and older: brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or doctor.
children under 6 years of age:
- instruct in good brushing and rinsing habits (to minimize swallowing).
- supervise children as necessary until capable of using without supervision.
- do not swallow
children under 2 years of age:
consult a dentist or doctor
INACTIVE INGREDIENT SECTION
INACTIVE INGREDIENTS:
water, hydrated silica, stabilized chlorine dioxide, sarkosyl (coconut derived), disodium hydrogen phosphate, cellulose gum, titanium dioxide, peppermint oil, sodium dihydrogen phosphate, sucralose, menthol crystals
SPL UNCLASSIFIED SECTION
Use of stabilized chlorine dioxide is protected under U.S. & foregin patents- see CloSYS.com/patents
OTC - QUESTIONS SECTION
Questions or comments?
Call 1-800-643-3337
