Omidria
These highlights do not include all the information needed to use OMIDRIA safely and effectively. See full prescribing information for OMIDRIA. OMIDRIA (phenylephrine and ketorolac intraocular solution) 1% / 0.3%, for addition to ocular irrigating solution Initial U.S. Approval: 2014
f25d7a42-da2a-7310-e053-2995a90a590f
HUMAN PRESCRIPTION DRUG LABEL
Jul 7, 2023
Rayner Surgical Inc.
DUNS: 118502278
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
phenylephrine and ketorolac
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Drug Labeling Information
DESCRIPTION SECTION
11 DESCRIPTION
Omidria is a sterile aqueous solution, containing the α 1-adrenergic receptor agonist phenylephrine HCl and the nonsteroidal anti-inflammatory ketorolac tromethamine, for addition to ocular irrigating solution.
The descriptions and structural formulae are:
Phenylephrine Hydrochloride Drug Substance:
|
Common Name: |
phenylephrine hydrochloride |
|
Chemical Name: |
(-)- m-Hydroxy-α-[(methylamino)methyl]benzyl alcohol hydrochloride |
|
Molecular Formula: |
C 9H 13NO 2 · HCl |
|
Molecular Weight: |
203.67 g/mole |
Figure 1: Chemical Structure for Phenylephrine HCl
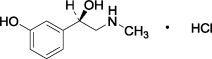
Ketorolac Tromethamine Drug Substance:
|
Common Name: |
ketorolac tromethamine |
|
Chemical Name: |
(±)-5-Benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid : 2-amino-2-(hydroxymethyl)-1,3-propanediol (1:1) |
|
Molecular Formula: |
C 15H 13NO 3 · C 4H 11NO 3 |
|
Molecular Weight: |
376.40 g/mole |
Figure 2: Chemical Structure for Ketorolac Tromethamine
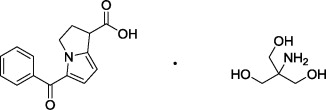
Omidria is a clear, colorless to slightly yellow, sterile solution concentrate with a pH of approximately 6.3.
Each vial of Omidria contains:
Actives: phenylephrine hydrochloride 12.4 mg/mL equivalent to 10.16 mg/mL of phenylephrine and ketorolac tromethamine 4.24 mg/mL equivalent to 2.88 mg/mL of ketorolac.
Inactives: citric acid monohydrate; sodium citrate dihydrate; water for injection; may include sodium hydroxide and/or hydrochloric acid for pH adjustment.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The two active pharmaceutical ingredients (API) in Omidria, phenylephrine and ketorolac, act to maintain pupil size by preventing intraoperative miosis, and reducing postoperative pain.
Phenylephrine is an α 1-adrenergic receptor agonist and, in the eye, acts as a mydriatic agent by contracting the radial muscle of the iris. Ketorolac is a nonsteroidal anti-inflammatory that inhibits both cyclooxygenase enzymes (COX-1 and COX-2), resulting in a decrease in tissue concentrations of prostaglandins to reduce pain due to surgical trauma. Ketorolac, by inhibiting prostaglandin synthesis secondary to ocular surgical insult or direct mechanical stimulation of the iris, also prevents surgically induced miosis.
12.3 Pharmacokinetics
In a pharmacokinetic study evaluating Omidria, systemic exposure to both phenylephrine and ketorolac was low or undetectable.
A single-dose of Omidria as part of the irrigation solution was administered in 14 patients during lens replacement surgery. The volume of irrigation solution used during surgery ranged between 150 mL to 300 mL (median 212.5 mL). Detectable phenylephrine plasma concentrations were observed in one of 14 patients (range 1.2 to 1.4 ng/mL) during the first 2 hours after the initiation of Omidria administration. The observed phenylephrine plasma concentrations could not be distinguished from the preoperative administration of phenylephrine 2.5% ophthalmic solution prior to exposure to Omidria.
Ketorolac plasma concentrations were detected in 10 of 14 patients (range 1.0 to 4.2 ng/mL) during the first 8 hours after the initiation of Omidria administration. The maximum ketorolac concentration was 15 ng/mL at 24 hours after the initiation of Omidria administration, which may have been due to application of postoperative ketorolac ophthalmic solution.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
Studies in Adults
The efficacy and safety of Omidria were evaluated in two Phase 3, randomized, multicenter, double-masked, placebo-controlled clinical trials in 808 adult patients undergoing cataract surgery or intraocular lens replacement.
Patients were randomized to either Omidria or placebo. Patients were treated with preoperative topical mydriatic and anesthetic agents. Pupil diameter was measured throughout the surgical procedure. Postoperative pain was evaluated by self-administered 0-100 mm visual analog scales (VAS).
Mydriasis was maintained in the Omidria-treated groups while the placebo- treated groups experienced progressive constriction.
Figure 3: Intraoperative Pupil Diameter (mm) Change-from-Baseline
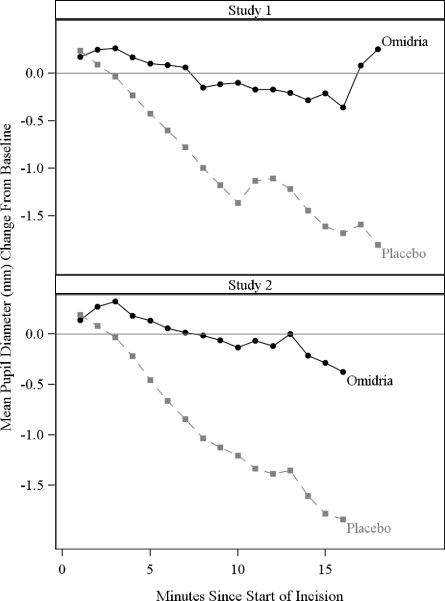
At the end of cortical clean-up, 23% of placebo-treated patients and 4% of Omidria-treated patients had a pupil diameter less than 6 mm (p < 0.01).
Pain during the initial 10-12 hours postoperatively was statistically significantly less in the Omidria-treated groups than in the placebo-treated groups.
Figure 4: Postoperative Mean Visual Analog Scale (VAS) Scores for Pain
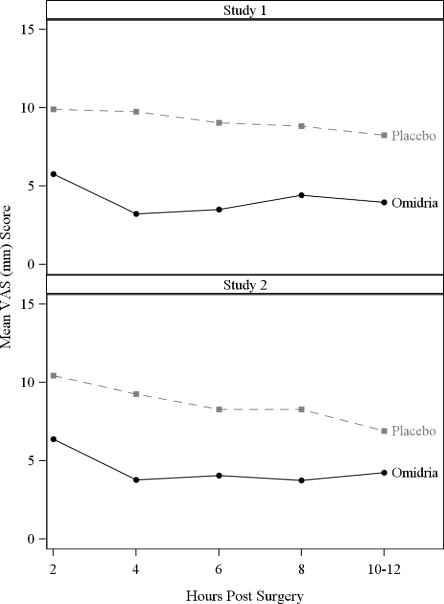
During the 10-12 hours postoperatively, 26% of Omidria-treated patients reported no pain (VAS = 0 at all timepoints) while 17% of placebo-treated patients reported no pain (p < 0.01).
Study in Pediatric Patients
The safety of Omidria was evaluated in a single, randomized, multicenter, double-masked, active-controlled clinical study in 72 pediatric patients up to 3 years old undergoing cataract surgery with or without intraocular lens replacement.
Patients were randomized to either Omidria or phenylephrine. Patients were treated with preoperative topical mydriatic and anesthetic agents. As in the adult studies, mydriasis was maintained in the Omidria-treated group. No overall differences in safety were observed between pediatric and adult patients.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Omidria (phenylephrine and ketorolac intraocular solution) 1%/0.3% is supplied in a clear, 5-mL glass, single-patient-use vial containing 4 mL of sterile solution, for addition to ocular irrigating solution.
Omidria is supplied in a multi-pack containing:
4 vials : NDC 82604-600-04 or
1 vials: NDC 82604-600-00
Storage: Store at 20˚ to 25˚C (68˚ to 77˚F). Protect from light.
